The Clostridium sporulation programs: diversity and preservation of endospore differentiation
- PMID: 25631287
- PMCID: PMC4402964
- DOI: 10.1128/MMBR.00025-14
The Clostridium sporulation programs: diversity and preservation of endospore differentiation
Abstract
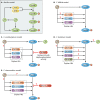
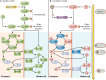
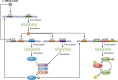
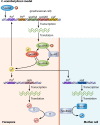
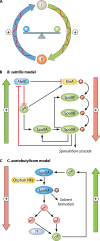
Bacillus and Clostridium organisms initiate the sporulation process when unfavorable conditions are detected. The sporulation process is a carefully orchestrated cascade of events at both the transcriptional and posttranslational levels involving a multitude of sigma factors, transcription factors, proteases, and phosphatases. Like Bacillus genomes, sequenced Clostridium genomes contain genes for all major sporulation-specific transcription and sigma factors (spo0A, sigH, sigF, sigE, sigG, and sigK) that orchestrate the sporulation program. However, recent studies have shown that there are substantial differences in the sporulation programs between the two genera as well as among different Clostridium species. First, in the absence of a Bacillus-like phosphorelay system, activation of Spo0A in Clostridium organisms is carried out by a number of orphan histidine kinases. Second, downstream of Spo0A, the transcriptional and posttranslational regulation of the canonical set of four sporulation-specific sigma factors (σ(F), σ(E), σ(G), and σ(K)) display different patterns, not only compared to Bacillus but also among Clostridium organisms. Finally, recent studies demonstrated that σ(K), the last sigma factor to be activated according to the Bacillus subtilis model, is involved in the very early stages of sporulation in Clostridium acetobutylicum, C. perfringens, and C. botulinum as well as in the very late stages of spore maturation in C. acetobutylicum. Despite profound differences in initiation, propagation, and orchestration of expression of spore morphogenetic components, these findings demonstrate not only the robustness of the endospore sporulation program but also the plasticity of the program to generate different complex phenotypes, some apparently regulated at the epigenetic level.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Bruggemann H, Baumer S, Fricke WF, Wiezer A, Liesegang H, Decker I, Herzberg C, Martinez-Arias R, Merkl R, Henne A, Gottschalk G. 2003. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc Natl Acad Sci U S A 100:1316–1321. doi: 10.1073/pnas.0335853100. - DOI - PMC - PubMed
-
- Sebaihia M, Peck MW, Minton NP, Thomson NR, Holden MTG, Mitchell WJ, Carter AT, Bentley SD, Mason DR, Crossman L, Paul CJ, Ivens A, Wells-Bennik MHJ, Davis IJ, Cerdeno-Tarraga AM, Churcher C, Quail MA, Chillingworth T, Feltwell T, Fraser A, Goodhead I, Hance Z, Jagels K, Larke N, Maddison M, Moule S, Mungall K, Norbertczak H, Rabbinowitsch E, Sanders M, Simmonds M, White B, Whithead S, Parkhill J. 2007. Genome sequence of a proteolytic (group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res 17:1082–1092. doi: 10.1101/gr.6282807. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

