Heterologous production of curcuminoids
- PMID: 25631288
- PMCID: PMC4402967
- DOI: 10.1128/MMBR.00031-14
Heterologous production of curcuminoids
Abstract
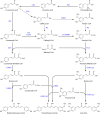
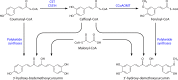
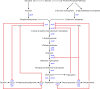
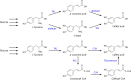
Curcuminoids, components of the rhizome of turmeric, show several beneficial biological activities, including anticarcinogenic, antioxidant, anti-inflammatory, and antitumor activities. Despite their numerous pharmaceutically important properties, the low natural abundance of curcuminoids represents a major drawback for their use as therapeutic agents. Therefore, they represent attractive targets for heterologous production and metabolic engineering. The understanding of biosynthesis of curcuminoids in turmeric made remarkable advances in the last decade, and as a result, several efforts to produce them in heterologous organisms have been reported. The artificial biosynthetic pathway (e.g., in Escherichia coli) can start with the supplementation of the amino acid tyrosine or phenylalanine or of carboxylic acids and lead to the production of several natural curcuminoids. Unnatural carboxylic acids can also be supplemented as precursors and lead to the production of unnatural compounds with possibly novel therapeutic properties. In this paper, we review the natural conversion of curcuminoids in turmeric and their production by E. coli using an artificial biosynthetic pathway. We also explore the potential of other enzymes discovered recently or already used in other similar biosynthetic pathways, such as flavonoids and stilbenoids, to increase curcuminoid yield and activity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. 2005. Chemistry and biological activities of C. longa. Trends Food Sci Technol 16:533–548. doi:10.1016/j.tifs.2005.08.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

