Controlled cord traction for the third stage of labour
- PMID: 25631379
- PMCID: PMC6464177
- DOI: 10.1002/14651858.CD008020.pub2
Controlled cord traction for the third stage of labour
Abstract
Background: Active management of the third stage of labour (AMTSL) consists of a group of interventions, including administration of a prophylactic uterotonic (at at or after delivery of the baby), baby, cord clamping and cutting, controlled cord traction (CCT) to deliver the placenta, and uterine massage. Recent recommendations are to delay cord clamping until the caregiver is ready to initiate CCT. The package of AMTSL reduces the risk of postpartum haemorrhage, (PPH), as does one component, routine use of uterotonics. The contribution, if any, of CCT needs to be quantified, as it is uncomfortable, and women may prefer a 'hands-off' approach. In addition its implementation has resource implications in terms of training of healthcare providers.
Objectives: To evaluate the effects of controlled cord traction during the third stage of labour, either with or without conventional active management.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (29 January 2014), PubMed (1966 to 29 January 2014), and reference lists of retrieved studies.
Selection criteria: Randomised controlled trials comparing planned CCT versus no planned CCT in women giving birth vaginally.
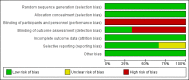
Data collection and analysis: Two authors assessed trial quality and extracted data using a standard data extraction form.
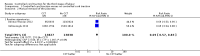
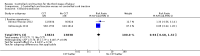
Main results: We included three methodologically sound trials with data on 199, 4058 and 23,616 women respectively. Blinding was not possible, but bias could be limited by the fact that blood loss was measured objectively.There was no difference in the risk of blood loss ≥ 1000 mL (three trials, 27,454 women; risk ratio (RR) 0.91, 95% confidence interval (CI) 0.77 to 1.08). Manual removal of the placenta was reduced with CCT (two trials, 27,665 women; RR 0.69, 95% CI 0.57 to 0.83). In the World Health Organization (WHO) trial the reduction in manual removal occurred mainly in sites where ergometrine was used routinely in the third stage of labour. The non-prespecified analysis excluding sites routinely using ergometrine for management of the third stage of labour found no difference in the risk of manual removal of the placenta in the WHO trial (one trial, 23,010 women; RR 1.03, 95% CI 0.73 to 1.46). The policy of restricting the third stage of labour to 30 minutes (4057 women; RR 0.69, 95% CI 0.53 to 0.90) may have had an effect in the French study.Among the secondary outcomes, there were reductions in blood loss ≥ 500 mL (three trials, 27,454 women; RR 0.93, 95% CI 0.88 to 0.99), mean blood loss (two trials, 27,255 women; mean difference (MD) -10.85 mL, 95% CI -16.73 to -4.98), and duration of the third stage of labour (two trials, 27,360 women; standardised MD -0.57, -0.59 to -0.54). There were no clear differences in use of additional uterotonics (three trials, 27,829 women; average RR 0.95, 95% CI 0.88 to 1.02), blood transfusion, maternal death/severe morbidity, operative procedures nor maternal satisfaction. Maternal pain (non-prespecified) was reduced in one trial (3760 women; RR 0.78, 95% CI 0.61 to 0.99).The following secondary outcomes were not reported upon in any of the trials: retained placenta for more than 60 minutes or as defined by trial author; maternal haemoglobin less than 9 g/dL at 24 to 48 hours post-delivery or blood transfusion; organ failure; intensive care unit admission; caregiver satisfaction; cost-effectiveness; evacuation of retained products; or infection.
Authors' conclusions: CCT has the advantage of reducing the risk of manual removal of the placenta in some circumstances, and evidence suggests that CCT can be routinely offered during the third stage of labour, provided the birth attendant has the necessary skills. CCT should remain a core competence of skilled birth attendants. However, the limited benefits of CCT in terms of severe PPH would not justify the major investment which would be needed to provide training in CCT skills for birth attendants who do not have formal training. Women who prefer a less interventional approach to management of the third stage of labour can be reassured that when a uterotonic agent is used, routine use of CCT can be omitted from the 'active management' package without increased risk of severe PPH, but that the risk of manual removal of the placenta may be increased.Research gaps include the use of CCT in the absence of a uterotonic, and the place of uterine massage in the management of the third stage of labour.
Conflict of interest statement
GJH, AMG and NM participated in a multicentre clinical trial of controlled cord traction (Gülmezoglu 2012). Decisions regarding the inclusion and interpretation of this trial were checked independently by a Research Associate working for the Cochrane Pregnancy and Childbirth Group.
GJH receives royalties from UpToDate for chapters related to
Figures
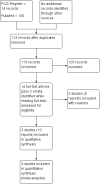























Update of
References
References to studies included in this review
Althabe 2009 {published data only}
Deneux‐Tharaux 2012 {published data only}
-
- Deneux‐Tharaux C, Sentilhes L, Maillard F, Closset E, Vardon D, Lepercq J, et al. Effect of controlled traction of the cord during the third stage of labour on the incidence of postpartum haemorrhage (Tracor study): A multicentre randomised controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):5‐6.
-
- Deneux‐Tharaux C, Sentilhes L, Maillard F, Closset E, Vardon D, Lepercq J, et al. Effect of controlled traction of the cord during the third stage of labour on the incidence of postpartum haemorrhage (Tracor study): a multicentre randomised controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):94.
Gülmezoglu 2012 {published data only}
-
- Armbruster D. Update on active management of the third stage of labour‐new data from the 2012 WHO trial. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S166.
-
- Gulmezoglu AM, Lumbiganon P, Landoulsi S, Widmer M, Abdel‐Aleem H, Festin M, et al. Active management of the third stage of labour with and without controlled cord traction: a randomised, controlled, non‐inferiority trial. [Erratum appears in Lancet. 2012 May 5;379(9827):1704]. Lancet 2012;379(9827):1721‐7. - PubMed
References to studies excluded from this review
Artymuk 2014 {published data only}
-
- Artymuk N, Surina M, Marochko T. Active management of the third stage of labor with and without controlled cord traction. International Journal of Gynecology and Obstetrics 2014;124(1):84‐5. - PubMed
-
- Artymuk NV, Surina MN, Kolesnikova NB, Marochko TY. Active management of the third stage of labor with and without controlled cord traction: A randomized controlled study. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S284‐S285. - PubMed
Bonham 1963 {published data only}
Kemp 1971 {published data only}
-
- Kemp J. A review of cord traction in the third stage of labour from 1963 to 1969. Medical Journal of Australia 1971;1(17):899‐903. - PubMed
Khan 1997 {published data only}
-
- Khan GQ, John IS, Wani S, Doherty T, Sibai BM. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. American Journal of Obstetrics and Gynecology 1997;177(4):770‐4. - PubMed
Sharma 2005 {published data only}
-
- Sharma JB, Pundir P, Malhotra M, Arora R. Evaluation of placental drainage as a method of placental delivery in vaginal deliveries. Archives of Gynecology and Obstetrics 2005;271(4):343‐5. - PubMed
Additional references
Anorlu 2008
Begley 2011
Bouvier‐Colle 2001
-
- Bouvier‐Colle MH, Ould EJ, Varnoux N, Goffinet F, Alexander S, Bayoumeu F, et al. Evaluation of the quality of care for severe obstetrical haemorrhage in three French regions. BJOG: an international journal of obstetrics and gynaecology 2001;108(9):898‐903. - PubMed
Brandt 1933
-
- Brandt ML. The mechanism and management of the third stage of labour. American Journal of Obstetrics and Gynecology 1933;25:662‐7.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICM 2003
-
- International Confederation of Midwives (ICM), International Federation of Gynaecologists and Obstetricians (FIGO). International joint policy statement: management of the third stage of labour to prevent postpartum hemorrhage. Journal of Obstetrics and Gynaecology Canada: JOGC 2003;25:952‐3. - PubMed
McDonald 2013
Mousa 2007
Rabe 2012
-
- Rabe H, Diaz‐Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD003248.pub3] - DOI - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Spencer 1962
Stearn 1963
-
- Stearn RH. Cord traction in the management of the third stage of labour. Suid‐Afrikaanse Tydskrif vir Obstetrie en Ginekologie 1963;37:925‐6. - PubMed
WHO 2007
-
- Mathai M, Gülmezoglu AM, Hill S. WHO recommendations for the prevention of postpartum haemorrhage. www.who.int/making_pregnancy_safer/documents/who_mps_0706/en/index.html (accessed January 2009). - PMC - PubMed
Winter 2007
-
- Winter C, Macfarlane A, Deneux‐Tharaux C, Zhang WZ, Alexander S, Brocklehurst P, et al. Variations in policies for management of the third stage of labour and the immediate management of postpartum haemorrhage in Europe. BJOG: an international journal of obstetrics and gynaecology 2007;114(7):845‐54. - PMC - PubMed
Zhang 2005
-
- Zhang WH, Alexander S, Bouvier‐Colle MH, Macfarlane A, MOMS‐B Group. Incidence of severe pre‐eclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population‐based study: the MOMS‐B survey. BJOG: an international journal of obstetrics and gynaecology 2005;112(1):89‐96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

