Probing bis-Fe(IV) MauG: experimental evidence for the long-range charge-resonance model
- PMID: 25631460
- PMCID: PMC4363735
- DOI: 10.1002/anie.201410247
Probing bis-Fe(IV) MauG: experimental evidence for the long-range charge-resonance model
Abstract
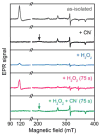
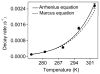
The biosynthesis of tryptophan tryptophylquinone, a protein-derived cofactor, involves a long-range reaction mediated by a bis-Fe(IV) intermediate of a diheme enzyme, MauG. Recently, a unique charge-resonance (CR) phenomenon was discovered in this intermediate, and a biological, long-distance CR model was proposed. This model suggests that the chemical nature of the bis-Fe(IV) species is not as simple as it appears; rather, it is composed of a collection of resonance structures in a dynamic equilibrium. Here, we experimentally evaluated the proposed CR model by introducing small molecules to, and measuring the temperature dependence of, bis-Fe(IV) MauG. Spectroscopic evidence was presented to demonstrate that the selected compounds increase the decay rate of the bis-Fe(IV) species by disrupting the equilibrium of the resonance structures that constitutes the proposed CR model. The results support this new CR model and bring a fresh concept to the classical CR theory.
Keywords: charge resonance; electronic structure; heme proteins; high-valence iron; near-infrared spectroscopy.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Badger B, Brocklehurst B. Nature. 1968;219:263. - PubMed
-
- Heckmann A, Lambert C. Angew Chem Int Ed. 2012;51:326–392;. - PubMed
- Agnew Chem. 2012;124:334–404.
-
- Kochi JK, Rathore R, Magueres PL. J Org Chem. 2000;65:6826–6836. - PubMed
-
- Takai A, Gros CP, Barbe JM, Guilard R, Fukuzumi S. Chem Eur J. 2009;15:3110–3122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

