AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress
- PMID: 25631767
- PMCID: PMC4375109
- DOI: 10.1182/blood-2014-08-594408
AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress
Abstract
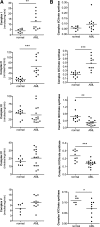
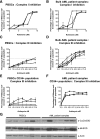
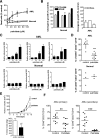
Mitochondrial respiration is a crucial component of cellular metabolism that can become dysregulated in cancer. Compared with normal hematopoietic cells, acute myeloid leukemia (AML) cells and patient samples have higher mitochondrial mass, without a concomitant increase in respiratory chain complex activity. Hence these cells have a lower spare reserve capacity in the respiratory chain and are more susceptible to oxidative stress. We therefore tested the effects of increasing the electron flux through the respiratory chain as a strategy to induce oxidative stress and cell death preferentially in AML cells. Treatment with the fatty acid palmitate induced oxidative stress and cell death in AML cells, and it suppressed tumor burden in leukemic cell lines and primary patient sample xenografts in the absence of overt toxicity to normal cells and organs. These data highlight a unique metabolic vulnerability in AML, and identify a new therapeutic strategy that targets abnormal oxidative metabolism in this malignancy.
© 2015 by The American Society of Hematology.
Figures




References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

