Expression of functional sphingosine-1 phosphate receptor-1 is reduced by B cell receptor signaling and increased by inhibition of PI3 kinase δ but not SYK or BTK in chronic lymphocytic leukemia cells
- PMID: 25632006
- PMCID: PMC4337486
- DOI: 10.4049/jimmunol.1402304
Expression of functional sphingosine-1 phosphate receptor-1 is reduced by B cell receptor signaling and increased by inhibition of PI3 kinase δ but not SYK or BTK in chronic lymphocytic leukemia cells
Abstract
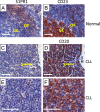
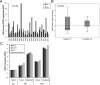
BCR signaling pathway inhibitors such as ibrutinib, idelalisib, and fostamatinib (respective inhibitors of Bruton's tyrosine kinase, PI3Kδ, and spleen tyrosine kinase) represent a significant therapeutic advance in B cell malignancies, including chronic lymphocytic leukemia (CLL). These drugs are distinctive in increasing blood lymphocytes while simultaneously shrinking enlarged lymph nodes, suggesting anatomical redistribution of CLL cells from lymph nodes into the blood. However, the mechanisms underlying this phenomenon are incompletely understood. In this study, we showed that the egress receptor, sphingosine-1-phosphate (S1P) receptor 1 (S1PR1), was expressed at low levels in normal germinal centers and CLL lymph nodes in vivo but became upregulated on normal B cells and, to a variable and lesser extent, CLL cells following in vitro incubation in S1P-free medium. Spontaneous recovery of S1PR1 expression on normal B and CLL cells was prevented by BCR cross-linking, whereas treatment of CLL cells with idelalisib increased S1PR1 expression and migration toward S1P, the greatest increase occurring in cases with unmutated IgH V region genes. Intriguingly, ibrutinib and fostamatinib had no effect on S1PR1 expression or function. Conversely, chemokine-induced migration, which requires integrin activation and is essential for the entry of lymphocytes into lymph nodes as well as their retention, was blocked by ibrutinib and fostamatinib, but not idelalisib. In summary, our results suggest that different BCR signaling inhibitors redistribute CLL cells from lymph nodes into the blood through distinct mechanisms: idelalisib actively promotes egress by upregulating S1PR1, whereas fostamatinib and ibrutinib may reduce CLL cell entry and retention by suppressing chemokine-induced integrin activation.
Copyright © 2015 The Authors.
Figures


Similar articles
-
The expression of sphingosine-1 phosphate receptor-1 in chronic lymphocytic leukemia cells is impaired by tumor microenvironmental signals and enhanced by piceatannol and R406.J Immunol. 2014 Sep 15;193(6):3165-74. doi: 10.4049/jimmunol.1400547. Epub 2014 Aug 15. J Immunol. 2014. PMID: 25127862
-
BCR signaling in chronic lymphocytic leukemia and related inhibitors currently in clinical studies.Int Rev Immunol. 2013 Aug;32(4):358-76. doi: 10.3109/08830185.2013.786711. Epub 2013 Apr 25. Int Rev Immunol. 2013. PMID: 23617253 Review.
-
Phosphatidylinositol 3-kinase δ blockade increases genomic instability in B cells.Nature. 2017 Feb 23;542(7642):489-493. doi: 10.1038/nature21406. Epub 2017 Feb 15. Nature. 2017. PMID: 28199309 Free PMC article.
-
BCR signaling inhibitors differ in their ability to overcome Mcl-1-mediated resistance of CLL B cells to ABT-199.Blood. 2016 Jun 23;127(25):3192-201. doi: 10.1182/blood-2015-10-675009. Epub 2016 Apr 19. Blood. 2016. PMID: 27095788
-
The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia.Haematologica. 2015 Dec;100(12):1495-507. doi: 10.3324/haematol.2014.119123. Haematologica. 2015. PMID: 26628631 Free PMC article. Review.
Cited by
-
Roles of PI3Kγ and PI3Kδ in mantle cell lymphoma proliferation and migration contributing to efficacy of the PI3Kγ/δ inhibitor duvelisib.Sci Rep. 2023 Mar 7;13(1):3793. doi: 10.1038/s41598-023-30148-3. Sci Rep. 2023. PMID: 36882482 Free PMC article.
-
Distinct roles for phosphoinositide 3-kinases γ and δ in malignant B cell migration.Leukemia. 2018 Sep;32(9):1958-1969. doi: 10.1038/s41375-018-0012-5. Epub 2018 Jan 31. Leukemia. 2018. PMID: 29479062 Free PMC article.
-
MicroRNAs: Potential prognostic and theranostic biomarkers in chronic lymphocytic leukemia.EJHaem. 2024 Feb 11;5(1):191-205. doi: 10.1002/jha2.849. eCollection 2024 Feb. EJHaem. 2024. PMID: 38406506 Free PMC article. Review.
-
Advancements on the Multifaceted Roles of Sphingolipids in Hematological Malignancies.Int J Mol Sci. 2022 Oct 22;23(21):12745. doi: 10.3390/ijms232112745. Int J Mol Sci. 2022. PMID: 36361536 Free PMC article. Review.
-
Role of microRNAs in Chronic Lymphocytic Leukemia.Int J Mol Sci. 2023 Aug 5;24(15):12471. doi: 10.3390/ijms241512471. Int J Mol Sci. 2023. PMID: 37569845 Free PMC article. Review.
References
-
- Stevenson F. K., Krysov S., Davies A. J., Steele A. J., Packham G. 2011. B-cell receptor signaling in chronic lymphocytic leukemia. Blood 118: 4313–4320. - PubMed
-
- Förster R., Schubel A., Breitfeld D., Kremmer E., Renner-Müller I., Wolf E., Lipp M. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99: 23–33. - PubMed
-
- Melchers F., Rolink A. G., Schaniel C. 1999. The role of chemokines in regulating cell migration during humoral immune responses. Cell 99: 351–354. - PubMed
-
- Till K. J., Coupland S. E., Pettitt A. R. 2014. Motility and trafficking in B-cell non-Hodgkin’s lymphoma (Review). Int. J. Oncol. 45: 5–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

