Collecting duct intercalated cell function and regulation
- PMID: 25632105
- PMCID: PMC4317747
- DOI: 10.2215/CJN.08880914
Collecting duct intercalated cell function and regulation
Abstract
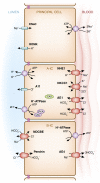
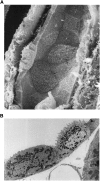
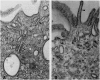
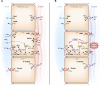
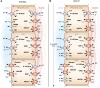
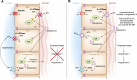
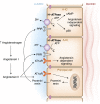
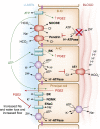
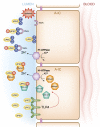
Intercalated cells are kidney tubule epithelial cells with important roles in the regulation of acid-base homeostasis. However, in recent years the understanding of the function of the intercalated cell has become greatly enhanced and has shaped a new model for how the distal segments of the kidney tubule integrate salt and water reabsorption, potassium homeostasis, and acid-base status. These cells appear in the late distal convoluted tubule or in the connecting segment, depending on the species. They are most abundant in the collecting duct, where they can be detected all the way from the cortex to the initial part of the inner medulla. Intercalated cells are interspersed among the more numerous segment-specific principal cells. There are three types of intercalated cells, each having distinct structures and expressing different ensembles of transport proteins that translate into very different functions in the processing of the urine. This review includes recent findings on how intercalated cells regulate their intracellular milieu and contribute to acid-base regulation and sodium, chloride, and potassium homeostasis, thus highlighting their potential role as targets for the treatment of hypertension. Their novel regulation by paracrine signals in the collecting duct is also discussed. Finally, this article addresses their role as part of the innate immune system of the kidney tubule.
Keywords: aldosterone; blood pressure; cell and transport; distal tubule; physiology; renal tubular acidosis.
Copyright © 2015 by the American Society of Nephrology.
Figures


References
-
- Crayen ML, Thoenes W: Architecture and cell structures in the distal nephron of the rat kidney. Cytobiologie 17: 197–211, 1978 - PubMed
-
- Davies JA, Davey MG: Collecting duct morphogenesis. Pediatr Nephrol 13: 535–541, 1999 - PubMed
-
- Meneton P, Loffing J, Warnock DG: Sodium and potassium handling by the aldosterone-sensitive distal nephron: The pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593–F601, 2004 - PubMed
-
- Madsen KM, Tisher CC: Structural-functional relationship along the distal nephron. Am J Physiol 250: F1–F15, 1986 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

