Visual landmark information gains control of the head direction signal at the lateral mammillary nuclei
- PMID: 25632114
- PMCID: PMC4308588
- DOI: 10.1523/JNEUROSCI.1418-14.2015
Visual landmark information gains control of the head direction signal at the lateral mammillary nuclei
Abstract
The neural representation of directional heading is conveyed by head direction (HD) cells located in an ascending circuit that includes projections from the lateral mammillary nuclei (LMN) to the anterodorsal thalamus (ADN) to the postsubiculum (PoS). The PoS provides return projections to LMN and ADN and is responsible for the landmark control of HD cells in ADN. However, the functional role of the PoS projection to LMN has not been tested. The present study recorded HD cells from LMN after bilateral PoS lesions to determine whether the PoS provides landmark control to LMN HD cells. After the lesion and implantation of electrodes, HD cell activity was recorded while rats navigated within a cylindrical arena containing a single visual landmark or while they navigated between familiar and novel arenas of a dual-chamber apparatus. PoS lesions disrupted the landmark control of HD cells and also disrupted the stability of the preferred firing direction of the cells in darkness. Furthermore, PoS lesions impaired the stable HD cell representation maintained by path integration mechanisms when the rat walked between familiar and novel arenas. These results suggest that visual information first gains control of the HD cell signal in the LMN, presumably via the direct PoS → LMN projection. This visual landmark information then controls HD cells throughout the HD cell circuit.
Keywords: landmark; mammillary; navigation; rat; spatial orientation; visual.
Copyright © 2015 the authors 0270-6474/15/351354-14$15.00/0.
Figures
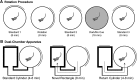
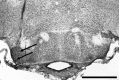
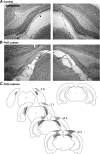




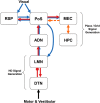
References
-
- Batschelet E. Circular statistics in biology. New York: Academic; 1981.
-
- Blair HT, Lipscomb BW, Sharp PE. Anticipatory time intervals of head-direction cells in the anterior thalamus of the rat: implications for path integration in the head-direction circuit. J Neurophysiol. 1997;78:145–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
