Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice
- PMID: 25632130
- PMCID: PMC4308600
- DOI: 10.1523/JNEUROSCI.2278-14.2015
Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice
Abstract
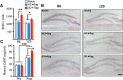
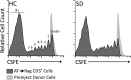
We examined whether cells of the adaptive immune system retain the memory of psychosocial stress and thereby alter mood states and CNS function in the host. Lymphocytes from mice undergoing chronic social defeat stress or from unstressed control mice were isolated and adoptively transferred into naive lymphopenic Rag2(-/-) mice. Changes in affective behavior, hippocampal cell proliferation, microglial activation states, and blood cytokine levels were examined in reconstituted stress-naive mice. The mice receiving lymphocytes from defeated donors showed less anxiety, more social behavior, and increased hippocampal cell proliferation compared with those receiving no cells or cells from unstressed donors. Mice receiving stressed immune cells had reduced pro-inflammatory cytokine levels in the blood relative to the other groups, an effect opposite to the elevated donor pro-inflammatory cytokine profile. Furthermore, mice receiving stressed immune cells had microglia skewed toward an anti-inflammatory, neuroprotective M2-like phenotype, an effect opposite the stressed donors' M1-like pro-inflammatory profile. However, stress had no effect on lymphocyte surface marker profiles in both donor and recipient mice. The data suggest that chronic stress-induced changes in the adaptive immune system, contrary to conferring anxiety and depressive behavior, protect against the deleterious effects of stress. Improvement in affective behavior is potentially mediated by reduced peripheral pro-inflammatory cytokine load, protective microglial activity, and increased hippocampal cell proliferation. The data identify the peripheral adaptive immune system as putatively involved in the mechanisms underlying stress resilience and a potential basis for developing novel rapid-acting antidepressant therapies.
Keywords: adaptive immune system; cytokines; depression; hippocampal neurogenesis; lymphocytes; stress.
Copyright © 2015 the authors 0270-6474/15/351530-09$15.00/0.
Figures

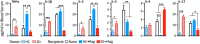

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
