Mechanism of neuromuscular dysfunction in Krabbe disease
- PMID: 25632136
- PMCID: PMC4308604
- DOI: 10.1523/JNEUROSCI.2431-14.2015
Mechanism of neuromuscular dysfunction in Krabbe disease
Abstract
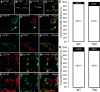
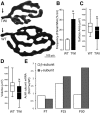
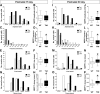
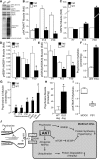
The atrophy of skeletal muscles in patients with Krabbe disease is a major debilitating manifestation that worsens their quality of life and limits the clinical efficacy of current therapies. The pathogenic mechanism triggering muscle wasting is unknown. This study examined structural, functional, and metabolic changes conducive to muscle degeneration in Krabbe disease using the murine (twitcher mouse) and canine [globoid cell leukodystrophy (GLD) dog] models. Muscle degeneration, denervation, neuromuscular [neuromuscular junction (NMJ)] abnormalities, and axonal death were investigated using the reporter transgenic twitcher-Thy1.1-yellow fluorescent protein mouse. We found that mutant muscles had significant numbers of smaller-sized muscle fibers, without signs of regeneration. Muscle growth was slow and weak in twitcher mice, with decreased maximum force. The NMJ had significant levels of activated caspase-3 but limited denervation. Mutant NMJ showed reduced surface areas and lower volumes of presynaptic terminals, with depressed nerve control, increased miniature endplate potential (MEPP) amplitude, decreased MEPP frequency, and increased rise and decay rate constants. Twitcher and GLD dog muscles had significant capacity to store psychosine, the neurotoxin that accumulates in Krabbe disease. Mechanistically, muscle defects involved the inactivation of the Akt pathway and activation of the proteasome pathway. Our work indicates that muscular dysfunction in Krabbe disease is compounded by a pathogenic mechanism involving at least the failure of NMJ function, activation of proteosome degradation, and a reduction of the Akt pathway. Akt, which is key for muscle function, may constitute a novel target to complement in therapies for Krabbe disease.
Keywords: Akt; Krabbe disease; neuromuscular junction; neuropathy; proteosome; psychosine.
Copyright © 2015 the authors 0270-6474/15/351606-11$15.00/0.
Figures



References
-
- Alden KJ, García J. Differential effect of gabapentin on neuronal and muscle calcium currents. J Pharmacol Exp Ther. 2001;297:727–735. - PubMed
-
- Bakshi R, Miletich RS, Kinkel PR, Emmet ML, Kinkel WR. High-resolution fluorodeoxyglucose positron emission tomography shows both global and regional cerebral hypometabolism in multiple sclerosis. J Neuroimaging. 1998;8:228–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
