Neural correlates of object-associated choice behavior in the perirhinal cortex of rats
- PMID: 25632144
- PMCID: PMC4308609
- DOI: 10.1523/JNEUROSCI.3160-14.2015
Neural correlates of object-associated choice behavior in the perirhinal cortex of rats
Abstract
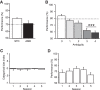
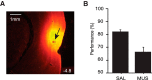
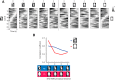
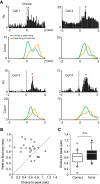
The perirhinal cortex (PRC) is reportedly important for object recognition memory, with supporting physiological evidence obtained largely from primate studies. Whether neurons in the rodent PRC also exhibit similar physiological correlates of object recognition, however, remains to be determined. We recorded single units from the PRC in a PRC-dependent, object-cued spatial choice task in which, when cued by an object image, the rat chose the associated spatial target from two identical discs appearing on a touchscreen monitor. The firing rates of PRC neurons were significantly modulated by critical events in the task, such as object sampling and choice response. Neuronal firing in the PRC was correlated primarily with the conjunctive relationships between an object and its associated choice response, although some neurons also responded to the choice response alone. However, we rarely observed a PRC neuron that represented a specific object exclusively regardless of spatial response in rats, although the neurons were influenced by the perceptual ambiguity of the object at the population level. Some PRC neurons fired maximally after a choice response, and this post-choice feedback signal significantly enhanced the neuronal specificity for the choice response in the subsequent trial. Our findings suggest that neurons in the rat PRC may not participate exclusively in object recognition memory but that their activity may be more dynamically modulated in conjunction with other variables, such as choice response and its outcomes.
Keywords: goal-directed task; object association; object recognition memory; perirhinal cortex; spatial memory.
Copyright © 2015 the authors 0270-6474/15/351692-14$15.00/0.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
