Morphometric and histologic substrates of cingulate integrity in elders with exceptional memory capacity
- PMID: 25632151
- PMCID: PMC4308613
- DOI: 10.1523/JNEUROSCI.2998-14.2015
Morphometric and histologic substrates of cingulate integrity in elders with exceptional memory capacity
Abstract
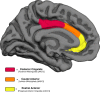
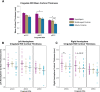
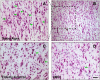
This human study is based on an established cohort of "SuperAgers," 80+-year-old individuals with episodic memory function at a level equal to, or better than, individuals 20-30 years younger. A preliminary investigation using structural brain imaging revealed a region of anterior cingulate cortex that was thicker in SuperAgers compared with healthy 50- to 65-year-olds. Here, we investigated the in vivo structural features of cingulate cortex in a larger sample of SuperAgers and conducted a histologic analysis of this region in postmortem specimens. A region-of-interest MRI structural analysis found cingulate cortex to be thinner in cognitively average 80+ year olds (n = 21) than in the healthy middle-aged group (n = 18). A region of the anterior cingulate cortex in the right hemisphere displayed greater thickness in SuperAgers (n = 31) compared with cognitively average 80+ year olds and also to the much younger healthy 50-60 year olds (p < 0.01). Postmortem investigations were conducted in the cingulate cortex in five SuperAgers, five cognitively average elderly individuals, and five individuals with amnestic mild cognitive impairment. Compared with other subject groups, SuperAgers showed a lower frequency of Alzheimer-type neurofibrillary tangles (p < 0.05). There were no differences in total neuronal size or count between subject groups. Interestingly, relative to total neuronal packing density, there was a higher density of von Economo neurons (p < 0.05), particularly in anterior cingulate regions of SuperAgers. These findings suggest that reduced vulnerability to the age-related emergence of Alzheimer pathology and higher von Economo neuron density in anterior cingulate cortex may represent biological correlates of high memory capacity in advanced old age.
Keywords: Alzheimer's pathology; aging; cingulate cortex; cognition; histology; structural MRI.
Copyright © 2015 the authors 0270-6474/15/351781-11$15.00/0.
Figures


References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG013854/AG/NIA NIH HHS/United States
- R01 AG045571/AG/NIA NIH HHS/United States
- F31-AG043270/AG/NIA NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- 5UL1RR025741/RR/NCRR NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- F31 AG043270/AG/NIA NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- AG045571/AG/NIA NIH HHS/United States
- 8UL1TR000150/TR/NCATS NIH HHS/United States
- AG13854/AG/NIA NIH HHS/United States
- R56 AG045571/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
