A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions
- PMID: 25632161
- PMCID: PMC4359927
- DOI: 10.1242/jcs.167544
A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions
Abstract
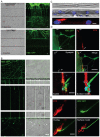
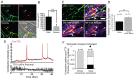
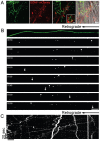
Bidirectional molecular communication between the motoneuron and the muscle is vital for neuromuscular junction (NMJ) formation and maintenance. The molecular mechanisms underlying such communication are of keen interest and could provide new targets for intervention in motoneuron disease. Here, we developed a microfluidic platform with motoneuron cell bodies on one side and muscle cells on the other, connected by motor axons extending through microgrooves to form functional NMJs. Using this system, we were able to differentiate between the proximal and distal effects of oxidative stress and glial-derived neurotrophic factor (GDNF), demonstrating a dying-back degeneration and retrograde transmission of pro-survival signaling, respectively. Furthermore, we show that GDNF acts differently on motoneuron axons versus soma, promoting axonal growth and innervation only when applied locally to axons. Finally, we track for the first time the retrograde transport of secreted GDNF from muscle to neuron. Thus, our data suggests spatially distinct effects of GDNF--facilitating growth and muscle innervation at axon terminals and survival pathways in the soma.
Keywords: Axon degeneration; Axonal transport; GDNF; Microfluidic chamber; Neuromuscular junction; Neurotrophic factors.
© 2015. Published by The Company of Biologists Ltd.
Figures




References
-
- Acsadi G., Anguelov R. A., Yang H., Toth G., Thomas R., Jani A., Wang Y., Ianakova E., Mohammad S., Lewis R. A. et al.(2002). Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum. Gene Ther. 13, 1047–1059 10.1089/104303402753812458 - DOI - PubMed
-
- Beauchamp J. R., Heslop L., Yu D. S., Tajbakhsh S., Kelly R. G., Wernig A., Buckingham M. E., Partridge T. A., Zammit P. S. (2000). Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 151, 1221–1234 10.1083/jcb.151.6.1221 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

