A crossover randomized comparative study of zofenopril and ramipril on cough reflex and airway inflammation in healthy volunteers
- PMID: 25632296
- PMCID: PMC4308941
- DOI: 10.1186/s12997-014-0007-5
A crossover randomized comparative study of zofenopril and ramipril on cough reflex and airway inflammation in healthy volunteers
Abstract
Background: Persistent dry cough is a well known unwanted effect of Angiotensin-Converting Enzyme inhibitors (ACE-i). Animal studies have shown that the ACE-i zofenopril has a less tussigenic effect compared to the widely used ACE-i ramipril. The aim of this study was to compare cough sensitivity to inhaled tussigens, as well as spontaneous cough in response to the administration of zofenopril and ramipril in healthy volunteers; pharmacokinetic (PK) data of both zofenopril and ramipril, as well as their respective active forms, zofenoprilat and ramiprilat, was also collected.
Methods: Forty healthy volunteers were enrolled in a randomized crossover study. Patients were administered zofenopril calcium salt (test drug) coated tablets, 30 mg daily dose or ramipril (reference drug) tablets, 10 mg daily dose, for 7 consecutive days in two periods separated by a 21-day wash-out period. Cough sensitivity to capsaicin and citric acid was assessed as the concentration of each tussigenic agent causing at least 2 (C2) or 5 coughs (C5); spontaneous cough was also monitored throughout the study. PK parameters of zofenopril, ramipril and their active forms, were collected for each of the two study periods. Airway inflammation, as assessed by fractional exhaled nitric oxide (FeNO) and bradykinin (BK) levels, were measured prior to and following each treatment period.
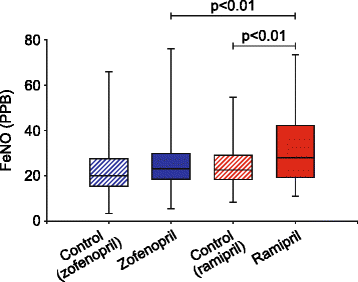
Results: Ramipril, but not zofenopril, increased (p < 0.01) cough sensitivity to both tussigenic agents as assessed by C2. With citric acid, C5 values calculated after both ramipril and zofenopril administration were significantly (p < 0.05 and p < 0.01, respectively) lower than corresponding control values. With both ACE-i drugs, spontaneous cough was infrequently reported by subjects. Zofenopril/zofenoprilat PK analysis showed higher area under the curve of plasma concentration, τ values (ng/ml x h) than ramipril/ramiprilat (zofenopril vs. ramipril, 84.25 ± 34.47 vs. 47.40 ± 21.30; and zofenoprilat vs. ramiprilat, 653.67 ± 174.91 vs. 182.26 ± 61.28). Both ACE-i drugs did not affect BK plasma levels; in contrast, ramipril, but not zofenopril, significantly increased control FeNO values (from 24 ± 9.6 parts per billion [PPB] to 33 ± 16 PPB; p < 0.01).
Conclusions: Zofenopril has a more favourable profile when compared to ramipril as shown by a reduced pro-inflammatory activity and less impact on the cough reflex.
Keywords: ACE-inhibitors; Airway inflammation; Cough; Ramipril; Zofenopril.
Figures



References
-
- Subissi A, Evangelista S, Giachetti A. Preclinical profile of zofenopril: an angiotensin converting enzyme inhibitor with peculiar cardioprotective properties. Cardiovasc Drug Rev. 1999;17:115–133. doi: 10.1111/j.1527-3466.1999.tb00008.x. - DOI
-
- Smith WH, Ball SG. Ramipril. Int J Clin Pract. 2000;54:255–260. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

