Function through bio-inspired, synthesis-informed design: step-economical syntheses of designed kinase inhibitors†Dedicated to Max Malacria, a friend and scholar whose science and creative contributions to step-economical synthesis have inspired us all and moved the field closer to the ideal.‡Electronic supplementary information (ESI) available: Synthetic procedures and spectral data. See DOI: 10.1039/c4qo00228hClick here for additional data file
- PMID: 25632347
- PMCID: PMC4304288
- DOI: 10.1039/c4qo00228h
Function through bio-inspired, synthesis-informed design: step-economical syntheses of designed kinase inhibitors†Dedicated to Max Malacria, a friend and scholar whose science and creative contributions to step-economical synthesis have inspired us all and moved the field closer to the ideal.‡Electronic supplementary information (ESI) available: Synthetic procedures and spectral data. See DOI: 10.1039/c4qo00228hClick here for additional data file
Abstract
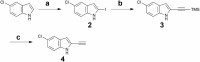
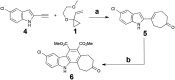
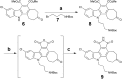
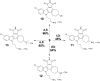
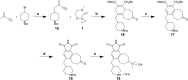
The human kinome comprises over 500 protein kinases. When mutated or over-expressed, many play critical roles in abnormal cellular functions associated with cancer, cardiovascular disease and neurological disorders. Here we report a step-economical approach to designed kinase inhibitors inspired by the potent, but non-selective, natural product staurosporine, and synthetically enabled by a novel, complexity-increasing, serialized [5 + 2]/[4 + 2] cycloaddition strategy. This function-oriented synthesis approach rapidly affords tunable scaffolds, and produced a low nanomolar inhibitor of protein kinase C.
Figures




References
-
- Bridges A. J. Chem. Rev. 2001;101:2541. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
