Targeting MKK3 as a novel anticancer strategy: molecular mechanisms and therapeutical implications
- PMID: 25633290
- PMCID: PMC4669782
- DOI: 10.1038/cddis.2014.591
Targeting MKK3 as a novel anticancer strategy: molecular mechanisms and therapeutical implications
Abstract
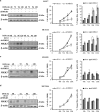
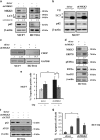
Mitogen-activated protein kinase kinase 3 (MAP2K3, MKK3) is a member of the dual specificity protein kinase group that belongs to the MAP kinase kinase family. This kinase is activated by mitogenic or stress-inducing stimuli and participates in the MAP kinase-mediated signaling cascade, leading to cell proliferation and survival. Several studies highlighted a critical role for MKK3 in tumor progression and invasion, and we previously identified MKK3 as transcriptional target of mutant (mut) p53 to sustain cell proliferation and survival, thus rendering MKK3 a promising target for anticancer therapies. Here, we found that targeting MKK3 with RNA interference, in both wild-type (wt) and mutp53-carrying cells, induced endoplasmic reticulum stress and autophagy that, respectively, contributed to stabilize wtp53 and degrade mutp53. MKK3 depletion reduced cancer cell proliferation and viability, whereas no significant effects were observed in normal cellular context. Noteworthy, MKK3 depletion in combination with chemotherapeutic agents increased tumor cell response to the drugs, in both wtp53 and mutp53 cancer cells, as demonstrated by enhanced poly (ADP-ribose) polymerase cleavage and reduced clonogenic ability in vitro. In addition, MKK3 depletion reduced tumor growth and improved biological response to chemotherapeutic in vivo. The overall results indicate MKK3 as a novel promising molecular target for the development of more efficient anticancer treatments in both wtp53- and mutp53-carrying tumors.
Figures




References
-
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–1912. - PubMed
-
- Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ et al. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J Biol Chem 1996; 271: 2886–2891. - PubMed
-
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 1995; 270: 7420–7426. - PubMed
-
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001; 22: 153–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

