The addition of recombinant vaccinia HER2/neu to oncolytic vaccinia-GMCSF given into the tumor microenvironment overcomes MDSC-mediated immune escape and systemic anergy
- PMID: 25633483
- PMCID: PMC4397129
- DOI: 10.1038/cgt.2015.2
The addition of recombinant vaccinia HER2/neu to oncolytic vaccinia-GMCSF given into the tumor microenvironment overcomes MDSC-mediated immune escape and systemic anergy
Abstract
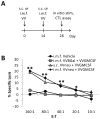
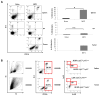
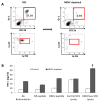
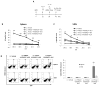
Effective immunotherapeutic strategies require the ability to generate a systemic antigen-specific response capable of impacting both primary and metastatic disease. We have built on our oncolytic vaccinia a granulocyte-macrophage colony-stimulating factor (GM-CSF) strategy by adding recombinant tumor antigen to increase the response in the tumor microenvironment and systemically. In the present study, orthotopic growth of a syngeneic HER2/neu-overexpressing mammary carcinoma in FVB/N mice (NBT1) was associated with increased Gr1(+)CD11b(+) myeloid-derived suppressor cells (MDSCs) both systemically and in the tumor microenvironment. This MDSC population had inhibitory effects on the HER2/neu-specific Th1 immune response. VVneu and VVGMCSF are recombinant oncolytic vaccinia viruses that encode HER2/neu and GM-CSF, respectively. Naive FVB mice vaccinated with combined VVneu and VVGMCSF given systemically developed systemic HER2/neu-specific immunity. NBT1-bearing mice became anergic to systemic immunization with combined VVneu and VVGMCSF. Intratumoral VVGMCSF failed to result in systemic antitumor immunity until combined with intratumoral VVneu. Infection/transfection of the tumor microenvironment with combined VVGMCSF and VVneu resulted in development of systemic tumor-specific immunity, reduction in splenic and tumor MDSC and therapeutic efficacy against tumors. These studies demonstrate the enhanced efficacy of oncolytic vaccinia virus recombinants encoding combined tumor antigen and GM-CSF in modulating the microenvironment of MDSC-rich tumors.
Conflict of interest statement
Dr. Lattime is an inventor of the patented recombinant Vaccinia-GMCSF which has been licensed to Sillajen and is being studied as JX-594. As such, he derives royalties and licensing fees from Thomas Jefferson University where the patent is held. Dr. de Vries and Dr. Monken have no conflicts.
Figures

Similar articles
-
Oncolytic and immunologic cancer therapy with GM-CSF-armed vaccinia virus of Tian Tan strain Guang9.Cancer Lett. 2016 Mar 28;372(2):251-7. doi: 10.1016/j.canlet.2016.01.025. Epub 2016 Jan 21. Cancer Lett. 2016. PMID: 26803055
-
Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma.Ann Surg Oncol. 2010 Mar;17(3):718-30. doi: 10.1245/s10434-009-0809-6. Ann Surg Oncol. 2010. PMID: 19915919 Clinical Trial.
-
Tumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances the Efficacy of Immune-Checkpoint Blockade.Clin Cancer Res. 2019 Mar 1;25(5):1612-1623. doi: 10.1158/1078-0432.CCR-18-1932. Epub 2018 Dec 11. Clin Cancer Res. 2019. PMID: 30538109
-
Arming oncolytic viruses to leverage antitumor immunity.Expert Opin Biol Ther. 2015 Jul;15(7):959-71. doi: 10.1517/14712598.2015.1044433. Epub 2015 May 10. Expert Opin Biol Ther. 2015. PMID: 25959450 Review.
-
[Oncolytic poxviruses].Mol Gen Mikrobiol Virusol. 2012;(1):8-15. Mol Gen Mikrobiol Virusol. 2012. PMID: 22702138 Review. Russian.
Cited by
-
Complex interplay between tumor microenvironment and cancer therapy.Front Med. 2018 Aug;12(4):426-439. doi: 10.1007/s11684-018-0663-7. Epub 2018 Aug 10. Front Med. 2018. PMID: 30097962 Review.
-
Immunosuppressive cells in oncolytic virotherapy for glioma: challenges and solutions.Front Cell Infect Microbiol. 2023 May 10;13:1141034. doi: 10.3389/fcimb.2023.1141034. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37234776 Free PMC article. Review.
-
High-throughput screening to enhance oncolytic virus immunotherapy.Oncolytic Virother. 2016 Apr 5;5:15-25. doi: 10.2147/OV.S66217. eCollection 2016. Oncolytic Virother. 2016. PMID: 27579293 Free PMC article. Review.
-
Oncolytic viruses: focusing on the tumor microenvironment.Cancer Gene Ther. 2015 Mar;22(4):169-71. doi: 10.1038/cgt.2015.11. Epub 2015 Feb 27. Cancer Gene Ther. 2015. PMID: 25721204 No abstract available.
-
Oncolytic virotherapy reverses the immunosuppressive tumor microenvironment and its potential in combination with immunotherapy.Cancer Cell Int. 2021 May 13;21(1):262. doi: 10.1186/s12935-021-01972-2. Cancer Cell Int. 2021. PMID: 33985527 Free PMC article. Review.
References
-
- Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421(6924):756–760. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature Reviews Molecular Cell Biology. 2001;2(2):127–137. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

