Glucose effectiveness in obese children: relation to degree of obesity and dysglycemia
- PMID: 25633663
- PMCID: PMC4370330
- DOI: 10.2337/dc14-2183
Glucose effectiveness in obese children: relation to degree of obesity and dysglycemia
Abstract
Objective: Impaired glucose effectiveness (GE) plays a role in the deterioration of glucose metabolism. Our aim was to validate a surrogate of GE derived from an oral glucose tolerance test (OGTT) and to assess the impact of degrees of obesity and of glucose tolerance on it.
Research design and methods: The OGTT-derived surrogate of GE (oGE) was validated in obese adolescents who underwent an OGTT and an intravenous glucose tolerance test (IVGTT). We then evaluated anthropometric determinants of the oGE and its impact on the dynamics of glucose tolerance in a cohort of children with varying degrees of obesity.
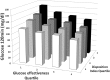
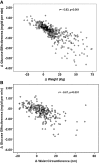
Results: The correlation of oGE and IVGTT-derived GE in 98 obese adolescents was r = 0.35 (P < 0.001) as a whole and r = 0.51 (P < 0.001) in subjects with normal glucose tolerance. In a cohort of 1,418 children, the adjusted GE was associated with increasing obesity (P < 0.001 for each category of obesity). Quartiles of oGE and the oral disposition index were associated with 2-h glucose levels (P < 0.001 for both). Among 421 nondiabetic obese subjects (276 subjects with normal glucose tolerance/145 subjects with impaired glucose tolerance who repeated their OGTT after a mean time of 28 ± 16 months), oGE changes were tightly associated with weight (r = 0.83, P < 0.001) and waist circumference changes (r = 0.67, P < 0.001). Baseline oGE and changes in oGE over time emerged as significant predictors of the change in 2-h glucose levels (standardized B = -0.76 and B = -0.98 respectively, P < 0.001 for both).
Conclusions: The oGE is associated with the degree of and changes in weight and waist circumference and is an independent predictor of glucose tolerance dynamics.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

References
-
- Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86 - PubMed
-
- Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 1996;19:1018–1030 - PubMed
-
- Nagasaka S, Tokuyama K, Kusaka I, et al. Endogenous glucose production and glucose effectiveness in type 2 diabetic subjects derived from stable-labeled minimal model approach. Diabetes 1999;48:1054–1060 - PubMed
-
- Basu A, Caumo A, Bettini F, et al. Impaired basal glucose effectiveness in NIDDM: contribution of defects in glucose disappearance and production, measured using an optimized minimal model independent protocol. Diabetes 1997;46:421–432 - PubMed
-
- Doi K, Taniguchi A, Nakai Y, et al. Decreased glucose effectiveness but not insulin resistance in glucose-tolerant offspring of Japanese non-insulin-dependent diabetic patients: a minimal-model analysis. Metabolism 1997;46:880–883 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK049230/DK/NIDDK NIH HHS/United States
- R01 HD028016/HD/NICHD NIH HHS/United States
- R01 HD040787/HD/NICHD NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- R01-HD-40787/HD/NICHD NIH HHS/United States
- UL1-RR-024139/RR/NCRR NIH HHS/United States
- DK-49230/DK/NIDDK NIH HHS/United States
- R01-HD-28016/HD/NICHD NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- DK-045735/DK/NIDDK NIH HHS/United States
- UL1 TR000142/TR/NCATS NIH HHS/United States
- UL1-RR-024134/RR/NCRR NIH HHS/United States
- K23 PA05143/PHS HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

