Nephrokeli, a Chinese herbal formula, may improve IgA nephropathy through regulation of the sphingosine-1-phosphate pathway
- PMID: 25633986
- PMCID: PMC4310606
- DOI: 10.1371/journal.pone.0116873
Nephrokeli, a Chinese herbal formula, may improve IgA nephropathy through regulation of the sphingosine-1-phosphate pathway
Abstract
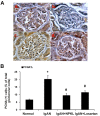
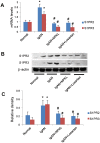
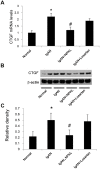
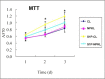
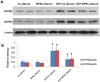
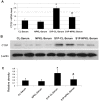
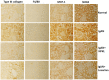
Nephrokeli (NPKL) is a Chinese herbal formula that has been used to treat patients with IgA nephropathy (IgAN) for improvement of proteinuria and kidney injury. However, the mechanism remains unclear. Sphingosine-1-phosphate (S1P) and its receptors S1PR2 and S1PR3 are known to play an important role in kidney disease. Here, we tested whether NPKL is able to regulate the S1P pathway in the kidney of IgAN rats. Four groups of rats were included in the study: Control, IgAN, IgAN treated with losartan, and IgAN treated with NPKL. The IgAN model was generated by injection of bovine serum albumin and staphylococcus enterotoxin B. We found that IgAN rats had increased staining for proliferating cell nuclear antigen (PCNA) in the mesangial area and increased mRNA and protein levels of S1PR2 and S1PR3 in the kidney compared to control rats. Connective tissue growth factor (CTGF), a downstream growth factor in the S1P pathway, was also elevated in the kidney of IgAN rats. Treatment with either NPKL or losartan was able to reduce PCNA staining and the expression of both S1PR2 and S1PR3 in the kidney of IgAN rats. However, NPKL (but not losartan treatment) reduced the expression of CTGF in the kidney of IgAN rats. In addition, we treated rat mesangial cells with sera collected from either NPKL-treated rats or control rats and found that NPKL-serum was able to reduce S1P-induced mesangial cell proliferation and the expression of S1PR2/S1PR3 and CTGF. NPKL also attenuates expression of fibrosis, inflammation, and oxidative stress markers in the kidney of IgAN rats. Our studies provide the mechanism by which NPKL attenuates kidney injury in IgAN rats.
Conflict of interest statement
Figures
Similar articles
-
Huangqi Guizhi Wuwu Decoction attenuates Podocyte cytoskeletal protein damage in IgA nephropathy rats by regulating AT1R/Nephrin/c-Abl pathway.Biomed Pharmacother. 2021 Oct;142:111907. doi: 10.1016/j.biopha.2021.111907. Epub 2021 Jul 30. Biomed Pharmacother. 2021. PMID: 34339916
-
Zhen-wu-tang protects against podocyte injury in rats with IgA nephropathy via PPARγ/NF-κB pathway.Biomed Pharmacother. 2018 May;101:635-647. doi: 10.1016/j.biopha.2018.02.127. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29518610
-
The study of Chinese medicinal herbal formula Shen San Fang in the treatment of experimental IgA nephropathy.Am J Chin Med. 2005;33(4):613-26. doi: 10.1142/S0192415X05003211. Am J Chin Med. 2005. PMID: 16173535
-
Targeting the sphingosine kinase/sphingosine 1-phosphate pathway to treat chronic inflammatory kidney diseases.Basic Clin Pharmacol Toxicol. 2014 Jan;114(1):44-9. doi: 10.1111/bcpt.12103. Epub 2013 Jul 15. Basic Clin Pharmacol Toxicol. 2014. PMID: 23789924 Review.
-
Sphingosine 1-phosphate in renal diseases.Cell Physiol Biochem. 2013;31(6):745-60. doi: 10.1159/000350093. Epub 2013 May 31. Cell Physiol Biochem. 2013. PMID: 23736205 Review.
Cited by
-
High-coverage targeted lipidomics could reveal lipid alterations and evaluate therapeutic efficacy of membranous nephropathy.Nutr Metab (Lond). 2022 Oct 12;19(1):68. doi: 10.1186/s12986-022-00701-4. Nutr Metab (Lond). 2022. PMID: 36224633 Free PMC article.
-
Oxidative Stress and Nutritional Antioxidants in Renal Diseases: A Narrative Review.Antioxidants (Basel). 2025 Jun 20;14(7):757. doi: 10.3390/antiox14070757. Antioxidants (Basel). 2025. PMID: 40722862 Free PMC article. Review.
-
Traditional Chinese Medicine in the Treatment of Chronic Kidney Diseases: Theories, Applications, and Mechanisms.Front Pharmacol. 2022 Jul 18;13:917975. doi: 10.3389/fphar.2022.917975. eCollection 2022. Front Pharmacol. 2022. PMID: 35924053 Free PMC article. Review.
-
Lipidomics reveals association of circulating lipids with body mass index and outcomes in IgA nephropathy patients.J Mol Cell Biol. 2021 Jul 17;13(8):565-75. doi: 10.1093/jmcb/mjab040. Online ahead of print. J Mol Cell Biol. 2021. PMID: 34272854 Free PMC article.
-
New Insights into the Pathogenesis of IgA Nephropathy.Kidney Dis (Basel). 2015 May;1(1):8-18. doi: 10.1159/000382134. Epub 2015 May 1. Kidney Dis (Basel). 2015. PMID: 26568951 Free PMC article.
References
-
- Widstam-Attorps U, Berg U, Bohman SO, Lefvert AK (1992) Proteinuria and renal function in relation to renal morphology. A clinicopathological study of IgA nephropathy at the time of kidney biopsy. Clin Nephrol 38: 245–253. - PubMed
-
- Coppo R, Amore A, Peruzzi L, Vergano L, Camilla R (2010) Innate immunity and IgA nephropathy. J Nephrol 23: 626–632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

