Tumor Endothelial Cells with Distinct Patterns of TGFβ-Driven Endothelial-to-Mesenchymal Transition
- PMID: 25634211
- PMCID: PMC4383705
- DOI: 10.1158/0008-5472.CAN-14-1616
Tumor Endothelial Cells with Distinct Patterns of TGFβ-Driven Endothelial-to-Mesenchymal Transition
Abstract
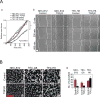
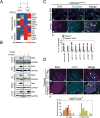
Endothelial-to-mesenchymal transition (EndMT) occurs during development and underlies the pathophysiology of multiple diseases. In tumors, unscheduled EndMT generates cancer-associated myofibroblasts that fuel inflammation and fibrosis, and may contribute to vascular dysfunction that promotes tumor progression. We report that freshly isolated subpopulations of tumor-specific endothelial cells (TEC) from a spontaneous mammary tumor model undergo distinct forms of EndMT in response to TGFβ stimulation. Although some TECs strikingly upregulate α smooth muscle actin (SMA), a principal marker of EndMT and activated myofibroblasts, counterpart normal mammary gland endothelial cells (NEC) showed little change in SMA expression after TGFβ treatment. Compared with NECs, SMA(+) TECs were 40% less motile in wound-healing assays and formed more stable vascular-like networks in vitro when challenged with TGFβ. Lineage tracing using ZsGreen(Cdh5-Cre) reporter mice confirmed that only a fraction of vessels in breast tumors contain SMA(+) TECs, suggesting that not all endothelial cells (EC) respond identically to TGFβ in vivo. Indeed, examination of 84 TGFβ-regulated target genes revealed entirely different genetic signatures in TGFβ-stimulated NEC and TEC cultures. Finally, we found that basic FGF (bFGF) exerts potent inhibitory effects on many TGFβ-regulated genes but operates in tandem with TGFβ to upregulate others. ECs challenged with TGFβ secrete bFGF, which blocks SMA expression in secondary cultures, suggesting a cell-autonomous or lateral-inhibitory mechanism for impeding mesenchymal differentiation. Together, our results suggest that TGFβ-driven EndMT produces a spectrum of EC phenotypes with different functions that could underlie the plasticity and heterogeneity of the tumor vasculature.
©2015 American Association for Cancer Research.
Conflict of interest statement
THERE ARE NO CONFLICTS OF INTEREST TO DISCLOSE
Figures





References
-
- Bischoff J, Aikawa E. Progenitor Cells Confer Plasticity to Cardiac Valve Endothelium. J of Cardiovasc Trans Res. 2011 - PubMed
-
- Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–6. - PubMed
-
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. - PubMed
-
- Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

