Snake neurotoxin α-bungarotoxin is an antagonist at native GABA(A) receptors
- PMID: 25634239
- PMCID: PMC4398322
- DOI: 10.1016/j.neuropharm.2015.01.001
Snake neurotoxin α-bungarotoxin is an antagonist at native GABA(A) receptors
Abstract
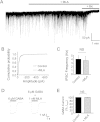
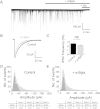
The snake neurotoxin α-bungarotoxin (α-Bgtx) is a competitive antagonist at nicotinic acetylcholine receptors (nAChRs) and is widely used to study their function and cell-surface expression. Increasingly, α-Bgtx is also used as an imaging tool for fluorophore-labelling studies, and given the structural conservation within the pentameric ligand-gated ion channel family, we assessed whether α-Bgtx could bind to recombinant and native γ-aminobutyric type-A receptors (GABAARs). Applying fluorophore-linked α-Bgtx to recombinant αxβ1/2γ2 GABAARs expressed in HEK-293 cells enabled clear cell-surface labelling of α2β1/2γ2 contrasting with the weaker staining of α1/4β1/2γ2, and no labelling for α3/5/6β1/2γ2. The labelling of α2β2γ2 was abolished by bicuculline, a competitive antagonist at GABAARs, and by d-tubocurarine (d-Tc), which acts in a similar manner at nAChRs and GABAARs. Labelling by α-Bgtx was also reduced by GABA, suggesting that the GABA binding site at the receptor β-α subunit interface forms part of the α-Bgtx binding site. Using whole-cell recording, high concentrations of α-Bgtx (20 μM) inhibited GABA-activated currents at all αxβ2γ2 receptors examined, but at lower concentrations (5 μM), α-Bgtx was selective for α2β2γ2. Using α-Bgtx, at low concentrations, permitted the selective inhibition of α2 subunit-containing GABAARs in hippocampal dentate gyrus granule cells, reducing synaptic current amplitudes without affecting the GABA-mediated tonic current. In conclusion, α-Bgtx can act as an inhibitor at recombinant and native GABAARs and may be used as a selective tool to inhibit phasic but not tonic currents in the hippocampus.
Keywords: Dentate gyrus; Electrophysiology; GABA receptor; Immunofluorescence; Nicotinic acetylcholine receptor; α-bungarotoxin.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures







References
-
- Aracri P., Consonni S., Morini R., Perrella M., Rodighiero S., Amadeo A., Becchetti A. Tonic modulation of GABA release by nicotinic acetylcholine receptors in layer V of the murine prefrontal cortex. Cereb. Cortex. 2010;20:1539–1555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

