Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients
- PMID: 25634360
- PMCID: PMC8153751
- DOI: 10.1016/j.jaut.2015.01.001
Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients
Abstract
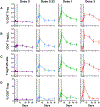
Most autoimmune diseases (AID) are linked to an imbalance between autoreactive effector T cells (Teffs) and regulatory T cells (Tregs). While blocking Teffs with immunosuppression has long been the only therapeutic option, activating/expanding Tregs may achieve the same objective without the toxicity of immunosuppression. We showed that low-dose interleukin-2 (ld-IL-2) safely expands/activates Tregs in patients with AID, such HCV-induced vasculitis and Type 1 Diabetes (T1D). Here we analyzed the kinetics and dose-relationship of IL-2 effects on immune responses in T1D patients. Ld-IL-2 therapy induced a dose-dependent increase in CD4(+)Foxp3(+) and CD8(+)Foxp3(+) Treg numbers and proportions, the duration of which was markedly dose-dependent. Tregs expressed enhanced levels of activation markers, including CD25, GITR, CTLA-4 and basal pSTAT5, and retained a 20-fold higher sensitivity to IL-2 than Teff and NK cells. Plasma levels of regulatory cytokines were increased in a dose-dependent manner, while cytokines linked to Teff and Th17 inflammatory cells were mostly unchanged. Global transcriptome analyses showed a dose-dependent decrease in immune response signatures. At the highest dose, Teff responses against beta-cell antigens were suppressed in all 4 patients tested. These results inform of broader changes induced by ld-IL-2 beyond direct effects on Tregs, and relevant for further development of ld-IL-2 for therapy and prevention of T1D, and other autoimmune and inflammatory diseases.
Keywords: Immunopathology; Immunotherapy; Inflammation; Pharmacokinetics; Tolerance.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures






References
-
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol, 2010;10:490–500. - PubMed
-
- Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med, 2006;12:178–80. - PubMed
-
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol, 2010;11:7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

