Treatment of leptothrix cells with ultrapure water poses a threat to their viability
- PMID: 25634812
- PMCID: PMC4381217
- DOI: 10.3390/biology4010050
Treatment of leptothrix cells with ultrapure water poses a threat to their viability
Abstract
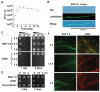
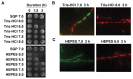
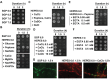
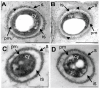
The genus Leptothrix, a type of Fe/Mn-oxidizing bacteria, is characterized by its formation of an extracellular and microtubular sheath. Although almost all sheaths harvested from natural aquatic environments are hollow, a few chained bacterial cells are occasionally seen within some sheaths of young stage. We previously reported that sheaths of Leptothrix sp. strain OUMS1 cultured in artificial media became hollow with aging due to spontaneous autolysis within the sheaths. In this study, we investigated environmental conditions that lead the OUMS1 cells to die. Treatment of the cells with ultrapure water or acidic buffers (pH 6.0) caused autolysis of the cells. Under these conditions, the plasma membrane and cytoplasm of cells were drastically damaged, resulting in leakage of intracellular electrolytes and relaxation of genomic DNA. The autolysis was suppressed by the presence of Ca2+. The hydrolysis of peptidoglycan by the lysozyme treatment similarly caused autolysis of the cells and was suppressed also by the presence of Ca2+. However, it remains unclear whether the acidic pH-dependent autolysis is attributable to damage of peptidoglycan. It was observed that L. discophora strain SP-6 cells also underwent autolysis when suspended in ultrapure water; it is however, uncertain whether this phenomenon is common among other members of the genus Leptothrix.
Figures


References
-
- Suzuki T., Ishihara H., Toyoda K., Shiraishi T., Kunoh H., Takada J. Autolysis of bacterial cells leads to formation of empty sheaths by Leptothrix spp. Minerals. 2013;3:247–257. doi: 10.3390/min3020247. - DOI
-
- Spring S. The general Leptothrix and Sphaerotilus. Prokaryotes. 2006;5:758–777.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

