Regulation of signal transduction by reactive oxygen species in the cardiovascular system
- PMID: 25634975
- PMCID: PMC4392388
- DOI: 10.1161/CIRCRESAHA.116.303584
Regulation of signal transduction by reactive oxygen species in the cardiovascular system
Abstract
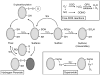
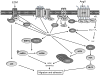
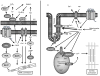
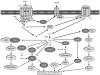
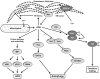
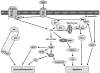
Oxidative stress has long been implicated in cardiovascular disease, but more recently, the role of reactive oxygen species (ROS) in normal physiological signaling has been elucidated. Signaling pathways modulated by ROS are complex and compartmentalized, and we are only beginning to identify the molecular modifications of specific targets. Here, we review the current literature on ROS signaling in the cardiovascular system, focusing on the role of ROS in normal physiology and how dysregulation of signaling circuits contributes to cardiovascular diseases, including atherosclerosis, ischemia-reperfusion injury, cardiomyopathy, and heart failure. In particular, we consider how ROS modulate signaling pathways related to phenotypic modulation, migration and adhesion, contractility, proliferation and hypertrophy, angiogenesis, endoplasmic reticulum stress, apoptosis, and senescence. Understanding the specific targets of ROS may guide the development of the next generation of ROS-modifying therapies to reduce morbidity and mortality associated with oxidative stress.
Keywords: cardiovascular diseases; oxidative stress; reactive oxygen species; signal transduction.
© 2015 American Heart Association, Inc.
Figures
References
-
- Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101:14D–19D. - PubMed
-
- Chandel NS, Tuveson DA. The promise and perils of antioxidants for cancer patients. N Engl J Med. 2014;371:177–178. - PubMed
-
- Fridovich I. Superoxide radical: An endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. - PubMed
-
- Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–13405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

