Protein structure. Structure and activity of tryptophan-rich TSPO proteins
- PMID: 25635100
- PMCID: PMC4341906
- DOI: 10.1126/science.aaa1534
Protein structure. Structure and activity of tryptophan-rich TSPO proteins
Abstract
Translocator proteins (TSPOs) bind steroids and porphyrins, and they are implicated in many human diseases, for which they serve as biomarkers and therapeutic targets. TSPOs have tryptophan-rich sequences that are highly conserved from bacteria to mammals. Here we report crystal structures for Bacillus cereus TSPO (BcTSPO) down to 1.7 Å resolution, including a complex with the benzodiazepine-like inhibitor PK11195. We also describe BcTSPO-mediated protoporphyrin IX (PpIX) reactions, including catalytic degradation to a previously undescribed heme derivative. We used structure-inspired mutations to investigate reaction mechanisms, and we showed that TSPOs from Xenopus and man have similar PpIX-directed activities. Although TSPOs have been regarded as transporters, the catalytic activity in PpIX degradation suggests physiological importance for TSPOs in protection against oxidative stress.
Copyright © 2015, American Association for the Advancement of Science.
Figures

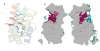
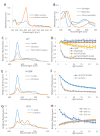
References
-
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. - PubMed
-
- Yeliseev AA, Kaplan S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1995;270:21167–21175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

