Clinical significance of macrophage phenotypes in cardiovascular disease
- PMID: 25635207
- PMCID: PMC4303745
- DOI: 10.1186/s40169-014-0042-1
Clinical significance of macrophage phenotypes in cardiovascular disease
Abstract
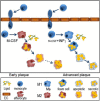
The emerging understanding of macrophage subsets and their functions in the atherosclerotic plaque has led to the consensus that M1 macrophages are pro-atherogenic while M2 macrophages may promote plaque stability, primarily though their tissue repair and anti-inflammatory properties. As such, modulating macrophage function to promote plaque stability is an exciting therapeutic prospect. This review will outline the involvement of the different macrophage subsets throughout atherosclerosis progression and in models of regression. It is evident that much of our understanding of macrophage function comes from in vitro or small animal models and, while such knowledge is valuable, we have much to learn about the roles of the macrophage subsets in the clinical setting in order to identify the key pathways to target to possibly promote plaque stability.
Keywords: Atherosclerosis; Cardiovascular disease; M1; M2; Macrophage; Plaque stability; Review.
Figures

Similar articles
-
Macrophage Subsets and Death Are Responsible for Atherosclerotic Plaque Formation.Front Immunol. 2022 Mar 30;13:843712. doi: 10.3389/fimmu.2022.843712. eCollection 2022. Front Immunol. 2022. PMID: 35432323 Free PMC article.
-
Macrophage Plasticity and Atherosclerosis Therapy.Front Mol Biosci. 2021 May 7;8:679797. doi: 10.3389/fmolb.2021.679797. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026849 Free PMC article. Review.
-
Understanding the role of alternative macrophage phenotypes in human atherosclerosis.Expert Rev Cardiovasc Ther. 2022 Sep;20(9):689-705. doi: 10.1080/14779072.2022.2111301. Epub 2022 Aug 10. Expert Rev Cardiovasc Ther. 2022. PMID: 35942866
-
Curcumin as a potential modulator of M1 and M2 macrophages: new insights in atherosclerosis therapy.Heart Fail Rev. 2019 May;24(3):399-409. doi: 10.1007/s10741-018-09764-z. Heart Fail Rev. 2019. PMID: 30673930 Review.
-
Characterizing the Role of Glycogen Synthase Kinase-3α/β in Macrophage Polarization and the Regulation of Pro-Atherogenic Pathways in Cultured Ldlr-/- Macrophages.Front Immunol. 2021 Jul 30;12:676752. doi: 10.3389/fimmu.2021.676752. eCollection 2021. Front Immunol. 2021. PMID: 34394077 Free PMC article.
Cited by
-
The relationship between preoperative serum cortisol level and the stability of plaque in carotid artery stenosis patients undergoing carotid endarterectomy.J Thorac Dis. 2016 Jul;8(7):1611-7. doi: 10.21037/jtd.2016.06.27. J Thorac Dis. 2016. PMID: 27499949 Free PMC article.
-
Progress in macrophage immune regulation of atherosclerosis.Am J Transl Res. 2025 May 15;17(5):3261-3275. doi: 10.62347/GMTC2479. eCollection 2025. Am J Transl Res. 2025. PMID: 40535630 Free PMC article. Review.
-
Macrophage subsets and their role: co-relation with colony-stimulating factor-1 receptor and clinical relevance.Immunol Res. 2023 Apr;71(2):130-152. doi: 10.1007/s12026-022-09330-8. Epub 2022 Oct 21. Immunol Res. 2023. PMID: 36266603 Free PMC article. Review.
-
Mechanical Stress Changes the Complex Interplay Between HO-1, Inflammation and Fibrosis, During Excisional Wound Repair.Front Med (Lausanne). 2015 Dec 15;2:86. doi: 10.3389/fmed.2015.00086. eCollection 2015. Front Med (Lausanne). 2015. PMID: 26697429 Free PMC article.
-
Regulation of macrophage polarization and plasticity by complex activation signals.Integr Biol (Camb). 2016 Sep 12;8(9):946-55. doi: 10.1039/c6ib00105j. Epub 2016 Aug 5. Integr Biol (Camb). 2016. PMID: 27492191 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
