Whole-body proton irradiation causes long-term damage to hematopoietic stem cells in mice
- PMID: 25635345
- PMCID: PMC4992474
- DOI: 10.1667/RR13887.1
Whole-body proton irradiation causes long-term damage to hematopoietic stem cells in mice
Abstract
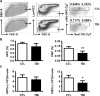
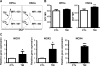
Space flight poses certain health risks to astronauts, including exposure to space radiation, with protons accounting for more than 80% of deep-space radiation. Proton radiation is also now being used with increasing frequency in the clinical setting to treat cancer. For these reasons, there is an urgent need to better understand the biological effects of proton radiation on the body. Such improved understanding could also lead to more accurate assessment of the potential health risks of proton radiation, as well as the development of improved strategies to prevent and mitigate its adverse effects. Previous studies have shown that exposure to low doses of protons is detrimental to mature leukocyte populations in peripheral blood, however, the underlying mechanisms are not known. Some of these detriments may be attributable to damage to hematopoietic stem cells (HSCs) that have the ability to self-renew, proliferate and differentiate into different lineages of blood cells through hematopoietic progenitor cells (HPCs). The goal of this study was to investigate the long-term effects of low-dose proton irradiation on HSCs. We exposed C57BL/6J mice to 1.0 Gy whole-body proton irradiation (150 MeV) and then studied the effects of proton radiation on HSCs and HPCs in the bone marrow (BM) 22 weeks after the exposure. The results showed that mice exposed to 1.0 Gy whole-body proton irradiation had a significant and persistent reduction of BM HSCs compared to unirradiated controls. In contrast, no significant changes were observed in BM HPCs after proton irradiation. Furthermore, irradiated HSCs and their progeny exhibited a significant impairment in clonogenic function, as revealed by the cobblestone area-forming cell (CAFC) and colony-forming cell assays, respectively. These long-term effects of proton irradiation on HSCs may be attributable to the induction of chronic oxidative stress in HSCs, because HSCs from irradiated mice exhibited a significant increase in NADPH oxidase 4 (NOX4) mRNA expression and reactive oxygen species (ROS) production. In addition, the increased production of ROS in HSCs was associated with a significant reduction in HSC quiescence and an increase in DNA damage. These findings indicate that exposure to proton radiation can lead to long-term HSC injury, probably in part by radiation-induced oxidative stress.
Figures



Similar articles
-
Low Doses of Oxygen Ion Irradiation Cause Acute Damage to Hematopoietic Cells in Mice.PLoS One. 2016 Jul 1;11(7):e0158097. doi: 10.1371/journal.pone.0158097. eCollection 2016. PLoS One. 2016. PMID: 27367604 Free PMC article.
-
28Si total body irradiation injures bone marrow hematopoietic stem cells via induction of cellular apoptosis.Life Sci Space Res (Amst). 2017 May;13:39-44. doi: 10.1016/j.lssr.2017.03.003. Epub 2017 Apr 5. Life Sci Space Res (Amst). 2017. PMID: 28554508 Free PMC article.
-
Low doses of oxygen ion irradiation cause long-term damage to bone marrow hematopoietic progenitor and stem cells in mice.PLoS One. 2017 Dec 12;12(12):e0189466. doi: 10.1371/journal.pone.0189466. eCollection 2017. PLoS One. 2017. PMID: 29232383 Free PMC article.
-
Hematopoietic stem cell injury induced by ionizing radiation.Antioxid Redox Signal. 2014 Mar 20;20(9):1447-62. doi: 10.1089/ars.2013.5635. Epub 2014 Feb 10. Antioxid Redox Signal. 2014. PMID: 24124731 Free PMC article. Review.
-
Protection of hematopoietic stem cells from stress-induced exhaustion and aging.Curr Opin Hematol. 2020 Jul;27(4):225-231. doi: 10.1097/MOH.0000000000000586. Curr Opin Hematol. 2020. PMID: 32398455 Review.
Cited by
-
HSCs transdifferentiate primarily to pneumonocytes in radiation-induced lung damage repair.Aging (Albany NY). 2021 Mar 3;13(6):8335-8354. doi: 10.18632/aging.202644. Epub 2021 Mar 3. Aging (Albany NY). 2021. PMID: 33686967 Free PMC article.
-
In vitro Assessment of the DNA Damage Response in Dental Mesenchymal Stromal Cells Following Low Dose X-ray Exposure.Front Public Health. 2021 Feb 15;9:584484. doi: 10.3389/fpubh.2021.584484. eCollection 2021. Front Public Health. 2021. PMID: 33692980 Free PMC article.
-
Osteoblast Derived Exosomes Alleviate Radiation- Induced Hematopoietic Injury.Front Bioeng Biotechnol. 2022 Apr 21;10:850303. doi: 10.3389/fbioe.2022.850303. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35528209 Free PMC article.
-
Total body proton and heavy-ion irradiation causes cellular senescence and promotes pro-osteoclastogenic activity in mouse bone marrow.Heliyon. 2021 Dec 29;8(1):e08691. doi: 10.1016/j.heliyon.2021.e08691. eCollection 2022 Jan. Heliyon. 2021. PMID: 35028468 Free PMC article.
-
Biological Effects of Scattered Versus Scanned Proton Beams on Normal Tissues in Total Body Irradiated Mice: Survival, Genotoxicity, Oxidative Stress and Inflammation.Antioxidants (Basel). 2020 Nov 24;9(12):1170. doi: 10.3390/antiox9121170. Antioxidants (Basel). 2020. PMID: 33255388 Free PMC article.
References
-
- Moore FD. Radiation burdens for humans on prolonged exomagnetospheric voyages. FASEB J. 1992;6:2338–43. - PubMed
-
- Townsend LW. Implications of the space radiation environment for human exploration in deep space. Radiat Prot Dosimetry. 2005;115:44–50. - PubMed
-
- Simonsen LC, Cucinotta FA, Atwell W, Nealy JE. Temporal analysis of the October 1989 proton flare using computerized anatomical models. Radiat Res. 1993;133:1–11. - PubMed
-
- Parsons JL, Townsend LW. Interplanetary crew dose rates for the August 1972 solar particle event. Radiat Res. 2000;153:729–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

