Tyrosine sulfation as a protein post-translational modification
- PMID: 25635379
- PMCID: PMC6272617
- DOI: 10.3390/molecules20022138
Tyrosine sulfation as a protein post-translational modification
Abstract
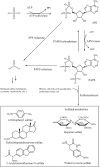
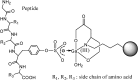
Integration of inorganic sulfate into biological molecules plays an important role in biological systems and is directly involved in the instigation of diseases. Protein tyrosine sulfation (PTS) is a common post-translational modification that was first reported in the literature fifty years ago. However, the significance of PTS under physiological conditions and its link to diseases have just begun to be appreciated in recent years. PTS is catalyzed by tyrosylprotein sulfotransferase (TPST) through transfer of an activated sulfate from 3'-phosphoadenosine-5'-phosphosulfate to tyrosine in a variety of proteins and peptides. Currently, only a small fraction of sulfated proteins is known and the understanding of the biological sulfation mechanisms is still in progress. In this review, we give an introductory and selective brief review of PTS and then summarize the basic biochemical information including the activity and the preparation of TPST, methods for the determination of PTS, and kinetics and reaction mechanism of TPST. This information is fundamental for the further exploration of the function of PTS that induces protein-protein interactions and the subsequent biochemical and physiological reactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Venkatachalam K.V. Human 3'-phosphoadenosine 5'-phosphosulfate (PAPS) synthase: Biochemistry, molecular biology and genetic deficiency. IUBMB Life. 2003;55:1–11. - PubMed
-
- Mulder G.J., Jakoby W.B. Sulfation. In: Mulder G.J., editor. Conjugation Reactions in Drug Metabolism. Taylor & Francis Ltd.; New York, NY, USA: 1990. pp. 107–161.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

