PCH-2 regulates Caenorhabditis elegans lifespan
- PMID: 25635513
- PMCID: PMC4350321
- DOI: 10.18632/aging.100713
PCH-2 regulates Caenorhabditis elegans lifespan
Abstract
Components or downstream targets of many signaling pathways such as Insulin/IGF-1 and TOR, as well as genes involved in cellular metabolism and bioenergetics can extend worm lifespan 20% or more. The C. elegans gene pch-2 and its homologs, including TRIP13 in humans, have been studied for their functions in cell mitosis and meiosis, but have never been implicated in lifespan regulation. Here we show that over-expression of TRIP13 in human fibroblasts confers resistance to environmental stressors such as UV radiation and oxidative stress. Furthermore, pch-2 overexpression in C. elegans extends worm lifespan, and enhances worm survival in response to various stressors. Conversely, reducing pch-2 expression with RNAi shortens worm lifespan. Additional genetic epistasis analysis indicates that the molecular mechanism of pch-2 in worm longevity is tied to functions of the sirtuin family, implying that pch-2 is another chromatin regulator for worm longevity. These findings suggest a novel function of the pch-2 gene involved in lifespan determination.
Conflict of interest statement
L.E.N. has a financial interest in Humacyte, Inc, a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
Figures
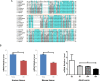




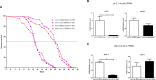

References
-
- Roeder GS, Bailis JM. The pachytene checkpoint. Trends Genet. 2000;16:395–403. - PubMed
-
- San-Segundo PA, Roeder GS. Pch2 links chromatin silencing to meiotic checkpoint control. Cell. 1999;97:313–324. - PubMed
-
- Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1–2.4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

