Role of p38 mitogen-activated protein kinase in vascular endothelial aging: interaction with Arginase-II and S6K1 signaling pathway
- PMID: 25635535
- PMCID: PMC4350325
- DOI: 10.18632/aging.100722
Role of p38 mitogen-activated protein kinase in vascular endothelial aging: interaction with Arginase-II and S6K1 signaling pathway
Abstract
p38 mitogen-activated protein kinase (p38) regulates cellular senescence and senescence-associated secretory phenotype (SASP), i.e., secretion of cytokines and/or chemokines. Previous work showed that augmented arginase-II (Arg-II) and S6K1 interact with each other to promote endothelial senescence through uncoupling of endothelial nitric oxide synthase (eNOS). Here we demonstrate eNOS-uncoupling, augmented expression/secretion of IL-6 and IL-8, elevation of p38 activation and Arg-II levels in senescent endothelial cells. Silencing Arg-II or p38α in senescent cells recouples eNOS and inhibits IL-6 and IL-8 secretion. Overexpression of Arg-II in young endothelial cells causes eNOS-uncoupling and enhances IL-6 and IL-8 expression/secretion, which is prevented by p38 inhibition or by antioxidant. Moreover, p38 activation and expression of IL-6 and KC (the murine IL-8 homologue) are increased in the heart and/or aortas of wild type (WT) old mice, which is abolished in mice with Arg-II gene deficiency (Arg-II-/-). In addition, inhibition of p38 in the old WT mice recouples eNOS function and reduces IL-6 and KC expression in the aortas and heart. Silencing Arg-II or p38a or S6K1 inhibits each other in senescence endothelial cells. Thus, Arg-II, p38, and S6K1 form a positive circuit which regulates endothelial senescence and cardiovascular aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
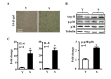
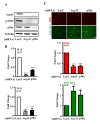
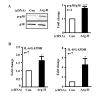
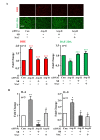
References
-
- Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. - PubMed
-
- Yepuri G, Velagapudi S, Xiong YY, Rajapakse AG, Montani JP, Ming XF, Yang ZH. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell. 2012;11:1005–1016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

