Cortical structure of hallucal metatarsals and locomotor adaptations in hominoids
- PMID: 25635768
- PMCID: PMC4311976
- DOI: 10.1371/journal.pone.0117905
Cortical structure of hallucal metatarsals and locomotor adaptations in hominoids
Abstract
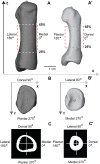
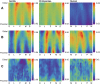
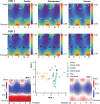
Diaphyseal morphology of long bones, in part, reflects in vivo loads experienced during the lifetime of an individual. The first metatarsal, as a cornerstone structure of the foot, presumably expresses diaphyseal morphology that reflects loading history of the foot during stance phase of gait. Human feet differ substantially from those of other apes in terms of loading histories when comparing the path of the center of pressure during stance phase, which reflects different weight transfer mechanisms. Here we use a novel approach for quantifying continuous thickness and cross-sectional geometric properties of long bones in order to test explicit hypotheses about loading histories and diaphyseal structure of adult chimpanzee, gorilla, and human first metatarsals. For each hallucal metatarsal, 17 cross sections were extracted at regularly-spaced intervals (2.5% length) between 25% and 65% length. Cortical thickness in cross sections was measured in one degree radially-arranged increments, while second moments of area were measured about neutral axes also in one degree radially-arranged increments. Standardized thicknesses and second moments of area were visualized using false color maps, while penalized discriminant analyses were used to evaluate quantitative species differences. Humans systematically exhibit the thinnest diaphyseal cortices, yet the greatest diaphyseal rigidities, particularly in dorsoplantar regions. Shifts in orientation of maximum second moments of area along the diaphysis also distinguish human hallucal metatarsals from those of chimpanzees and gorillas. Diaphyseal structure reflects different loading regimes, often in predictable ways, with human versus non-human differences probably resulting both from the use of arboreal substrates by non-human apes and by differing spatial relationships between hallux position and orientation of the substrate reaction resultant during stance. The novel morphological approach employed in this study offers the potential for transformative insights into form-function relationships in additional long bones, including those of extinct organisms (e.g., fossils).
Conflict of interest statement
Figures



Similar articles
-
Articular to diaphyseal proportions of human and great ape metatarsals.Am J Phys Anthropol. 2010 Oct;143(2):198-207. doi: 10.1002/ajpa.21306. Am J Phys Anthropol. 2010. PMID: 20853475
-
Mouse hallucal metatarsal cross-sectional geometry in a simulated fine branch niche.J Morphol. 2015 Jul;276(7):759-65. doi: 10.1002/jmor.20376. Epub 2015 Mar 11. J Morphol. 2015. PMID: 25758098
-
Metatarsal torsion in monkeys, apes, humans and australopiths.J Hum Evol. 2013 Jan;64(1):93-108. doi: 10.1016/j.jhevol.2012.10.008. Epub 2012 Dec 4. J Hum Evol. 2013. PMID: 23219163
-
Rethinking the evolution of the human foot: insights from experimental research.J Exp Biol. 2018 Sep 6;221(Pt 17):jeb174425. doi: 10.1242/jeb.174425. J Exp Biol. 2018. PMID: 30190415 Review.
-
Force, pressure, and motion measurements in the foot: current concepts.Clin Podiatr Med Surg. 1988 Jul;5(3):491-508. Clin Podiatr Med Surg. 1988. PMID: 3293751 Review.
Cited by
-
Ape femoral-humeral rigidities and arboreal locomotion.Am J Biol Anthropol. 2022 Dec;179(4):624-639. doi: 10.1002/ajpa.24632. Epub 2022 Oct 31. Am J Biol Anthropol. 2022. PMID: 36790629 Free PMC article.
-
Endostructural and periosteal growth of the human humerus.Anat Rec (Hoboken). 2023 Jan;306(1):60-78. doi: 10.1002/ar.25048. Epub 2022 Aug 25. Anat Rec (Hoboken). 2023. PMID: 36054304 Free PMC article.
-
Divergent locomotor evolution in "giant" kangaroos: Evidence from foot bone bending resistances and microanatomy.J Morphol. 2022 Mar;283(3):313-332. doi: 10.1002/jmor.21445. Epub 2022 Jan 18. J Morphol. 2022. PMID: 34997777 Free PMC article.
-
Cortical bone distribution of the proximal phalanges in great apes: implications for reconstructing manual behaviours.J Anat. 2023 Nov;243(5):707-728. doi: 10.1111/joa.13918. Epub 2023 Jun 26. J Anat. 2023. PMID: 37358024 Free PMC article.
-
Intensive terrestrial or marine locomotor strategies are associated with inter- and intra-limb bone functional adaptation in living female athletes.Am J Phys Anthropol. 2019 Mar;168(3):566-581. doi: 10.1002/ajpa.23773. Epub 2019 Jan 5. Am J Phys Anthropol. 2019. PMID: 30613942 Free PMC article.
References
-
- Gebo DL, Schwartz GT (2006) Foot bones from Omo: Implications for hominid evolution. Am J Phys Anthropol 129: 499–511. - PubMed
-
- Lewis OJ (1989) Functional morphology of the evolving hand and foot. Oxford: Clarendon Press;
-
- Gebo DL (1993) Functional morphology of the foot of primates In: Gebo D. L., editor editors. Postcranial adaptations in nonhuman primates. Dekalb: Northern Illinois University Press; pp. 175–198.
-
- Stern JT, Susman RL (1983) The locomotor anatomy of Australopithecus afarensis . Am J Phys Anthropol 60: 279–317. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

