Therapy with plasma purified alpha1-antitrypsin (Prolastin®) induces time-dependent changes in plasma levels of MMP-9 and MPO
- PMID: 25635861
- PMCID: PMC4311911
- DOI: 10.1371/journal.pone.0117497
Therapy with plasma purified alpha1-antitrypsin (Prolastin®) induces time-dependent changes in plasma levels of MMP-9 and MPO
Abstract
The common Z mutation (Glu342Lys) of α1-antitrypsin (A1AT) results in the polymerization and intracellular retention of A1AT protein. The concomitant deficiency of functional A1AT predisposes PiZZ subjects to early onset emphysema. Clinical studies have implied that, among the biomarkers associated with emphysema, matrix metalloproteinase 9 (MMP-9) is of particular importance. Increased plasma MMP-9 levels are proposed to predict the decline of lung function as well as greater COPD exacerbations in A1AT deficiency-associated emphysema. The aim of the present study was to investigate the effect of A1AT therapy (Prolastin) on plasma MMP-9 and myeloperoxidase (MPO) levels. In total 34 PiZZ emphysema patients were recruited: 12 patients without and 22 with weekly intravenous (60 mg/kg body weight) A1AT therapy. The quantitative analysis of A1AT, MMP-9 and MPO was performed in serum and in supernatants of blood neutrophils isolated from patients before and after therapy. Patients with Prolastin therapy showed significantly lower serum MMP-9 and MPO levels than those without therapy. However, parallel analysis revealed that a rapid infusion of Prolastin is accompanied by a transient elevation of plasma MMP-9 and MPO levels. Experiments with freshly isolated blood neutrophils confirmed that therapy with Prolastin causes transient MMP-9 and MPO release. Prolastin induced the rapid release of MMP-9 and MPO when added directly to neutrophil cultures and this reaction was associated with the presence of IgA in A1AT preparation. Our data support the conclusion that changes in plasma levels of MMP-9 and MPO mirror the effect of Prolastin on blood neutrophils.
Conflict of interest statement
Figures




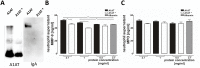
Similar articles
-
The effects of weekly augmentation therapy in patients with PiZZ α1-antitrypsin deficiency.Int J Chron Obstruct Pulmon Dis. 2012;7:687-96. doi: 10.2147/COPD.S34560. Epub 2012 Sep 28. Int J Chron Obstruct Pulmon Dis. 2012. PMID: 23055718 Free PMC article.
-
The fibrinogen cleavage product Aα-Val360, a specific marker of neutrophil elastase activity in vivo.Thorax. 2011 Aug;66(8):686-91. doi: 10.1136/thx.2010.154690. Epub 2011 May 26. Thorax. 2011. PMID: 21617168 Clinical Trial.
-
SPARTA clinical trial design: exploring the efficacy and safety of two dose regimens of alpha1-proteinase inhibitor augmentation therapy in alpha1-antitrypsin deficiency.Respir Med. 2015 Apr;109(4):490-9. doi: 10.1016/j.rmed.2015.01.022. Epub 2015 Feb 13. Respir Med. 2015. PMID: 25727857 Clinical Trial.
-
Alpha-1-antitrypsin augmentation therapy for alpha-1-antitrypsin deficiency.Am J Med. 1988 Jun 24;84(6A):52-62. doi: 10.1016/0002-9343(88)90159-3. Am J Med. 1988. PMID: 3289387 Review.
-
Well-Known and Less Well-Known Functions of Alpha-1 Antitrypsin. Its Role in Chronic Obstructive Pulmonary Disease and Other Disease Developments.Ann Am Thorac Soc. 2016 Aug;13 Suppl 4:S280-8. doi: 10.1513/AnnalsATS.201507-468KV. Ann Am Thorac Soc. 2016. PMID: 27564662 Review.
Cited by
-
Mechanisms behind Retinal Ganglion Cell Loss in Diabetes and Therapeutic Approach.Int J Mol Sci. 2020 Mar 28;21(7):2351. doi: 10.3390/ijms21072351. Int J Mol Sci. 2020. PMID: 32231131 Free PMC article. Review.
-
Effect of sevoflurane pretreatment in relieving liver ischemia/reperfusion-induced pulmonary and hepatic injury.Acta Cir Bras. 2019 Oct 14;34(8):e201900805. doi: 10.1590/s0102-865020190080000005. eCollection 2019. Acta Cir Bras. 2019. PMID: 31618405 Free PMC article.
-
Point Mutation of a Non-Elastase-Binding Site in Human α1-Antitrypsin Alters Its Anti-Inflammatory Properties.Front Immunol. 2018 May 1;9:759. doi: 10.3389/fimmu.2018.00759. eCollection 2018. Front Immunol. 2018. PMID: 29780379 Free PMC article.
-
BP180 Is Critical in the Autoimmunity of Bullous Pemphigoid.Front Immunol. 2017 Dec 8;8:1752. doi: 10.3389/fimmu.2017.01752. eCollection 2017. Front Immunol. 2017. PMID: 29276517 Free PMC article. Review.
-
The Multifaceted Effects of Alpha1-Antitrypsin on Neutrophil Functions.Front Pharmacol. 2018 Apr 17;9:341. doi: 10.3389/fphar.2018.00341. eCollection 2018. Front Pharmacol. 2018. PMID: 29719508 Free PMC article. Review.
References
-
- Janciauskiene S (2001) Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim Biophys Acta 1535: 221–235. - PubMed
-
- Blanco I, de Serres FJ, Fernandez-Bustillo E, Lara B, Miravitlles M (2006) Estimated numbers and prevalence of PI*S and PI*Z alleles of alpha1-antitrypsin deficiency in European countries. Eur Respir J 27: 77–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

