Therapy with plasma purified alpha1-antitrypsin (Prolastin®) induces time-dependent changes in plasma levels of MMP-9 and MPO
- PMID: 25635861
- PMCID: PMC4311911
- DOI: 10.1371/journal.pone.0117497
Therapy with plasma purified alpha1-antitrypsin (Prolastin®) induces time-dependent changes in plasma levels of MMP-9 and MPO
Abstract
The common Z mutation (Glu342Lys) of α1-antitrypsin (A1AT) results in the polymerization and intracellular retention of A1AT protein. The concomitant deficiency of functional A1AT predisposes PiZZ subjects to early onset emphysema. Clinical studies have implied that, among the biomarkers associated with emphysema, matrix metalloproteinase 9 (MMP-9) is of particular importance. Increased plasma MMP-9 levels are proposed to predict the decline of lung function as well as greater COPD exacerbations in A1AT deficiency-associated emphysema. The aim of the present study was to investigate the effect of A1AT therapy (Prolastin) on plasma MMP-9 and myeloperoxidase (MPO) levels. In total 34 PiZZ emphysema patients were recruited: 12 patients without and 22 with weekly intravenous (60 mg/kg body weight) A1AT therapy. The quantitative analysis of A1AT, MMP-9 and MPO was performed in serum and in supernatants of blood neutrophils isolated from patients before and after therapy. Patients with Prolastin therapy showed significantly lower serum MMP-9 and MPO levels than those without therapy. However, parallel analysis revealed that a rapid infusion of Prolastin is accompanied by a transient elevation of plasma MMP-9 and MPO levels. Experiments with freshly isolated blood neutrophils confirmed that therapy with Prolastin causes transient MMP-9 and MPO release. Prolastin induced the rapid release of MMP-9 and MPO when added directly to neutrophil cultures and this reaction was associated with the presence of IgA in A1AT preparation. Our data support the conclusion that changes in plasma levels of MMP-9 and MPO mirror the effect of Prolastin on blood neutrophils.
Conflict of interest statement
Figures




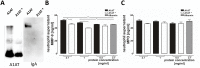
References
-
- Janciauskiene S (2001) Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim Biophys Acta 1535: 221–235. - PubMed
-
- Blanco I, de Serres FJ, Fernandez-Bustillo E, Lara B, Miravitlles M (2006) Estimated numbers and prevalence of PI*S and PI*Z alleles of alpha1-antitrypsin deficiency in European countries. Eur Respir J 27: 77–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

