Multidrug efflux pumps from Enterobacteriaceae, Vibrio cholerae and Staphylococcus aureus bacterial food pathogens
- PMID: 25635914
- PMCID: PMC4344678
- DOI: 10.3390/ijerph120201487
Multidrug efflux pumps from Enterobacteriaceae, Vibrio cholerae and Staphylococcus aureus bacterial food pathogens
Abstract
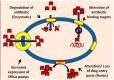
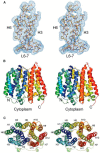
Foodborne illnesses caused by bacterial microorganisms are common worldwide and constitute a serious public health concern. In particular, microorganisms belonging to the Enterobacteriaceae and Vibrionaceae families of Gram-negative bacteria, and to the Staphylococcus genus of Gram-positive bacteria are important causative agents of food poisoning and infection in the gastrointestinal tract of humans. Recently, variants of these bacteria have developed resistance to medically important chemotherapeutic agents. Multidrug resistant Escherichia coli, Salmonella enterica, Vibrio cholerae, Enterobacter spp., and Staphylococcus aureus are becoming increasingly recalcitrant to clinical treatment in human patients. Of the various bacterial resistance mechanisms against antimicrobial agents, multidrug efflux pumps comprise a major cause of multiple drug resistance. These multidrug efflux pump systems reside in the biological membrane of the bacteria and actively extrude antimicrobial agents from bacterial cells. This review article summarizes the evolution of these bacterial drug efflux pump systems from a molecular biological standpoint and provides a framework for future work aimed at reducing the conditions that foster dissemination of these multidrug resistant causative agents through human populations.
Figures



References
-
- Nyenje M.E., Odjadjare C.E., Tanih N.F., Green E., Ndip R.N. Foodborne pathogens recovered from ready-to-eat foods from roadside cafeterias and retail outlets in Alice, Eastern Cape Province, South Africa: Public health implications. Int. J. Environ. Res. Public Health. 2012;9:2608–2619. doi: 10.3390/ijerph9082608. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

