Estimating the clinical and economic benefit associated with incremental improvements in sustained virologic response in chronic hepatitis C
- PMID: 25635922
- PMCID: PMC4311930
- DOI: 10.1371/journal.pone.0117334
Estimating the clinical and economic benefit associated with incremental improvements in sustained virologic response in chronic hepatitis C
Abstract
Introduction: Hepatitis C virus (HCV) infection is one of the principle causes of chronic liver disease. Successful treatment significantly decreases the risk of hepatic morbidity and mortality. Current standard of care achieves sustained virologic response (SVR) rates of 40-80%; however, the HCV therapy landscape is rapidly evolving. The objective of this study was to quantify the clinical and economic benefit associated with increasing levels of SVR.
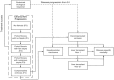
Methods: A published Markov model (MONARCH) that simulates the natural history of hepatitis C over a lifetime horizon was used. Discounted and non-discounted life-years (LYs), quality-adjusted life-years (QALYs) and cost of complication management were estimated for various plausible SVR rates. To demonstrate the robustness of projections obtained, the model was validated to ten UK-specific HCV studies.
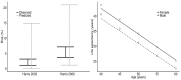
Results: QALY estimates ranged from 18.0 years for those treated successfully in fibrosis stage F0 to 7.5 years (discounted) for patients in fibrosis stage F4 who remain untreated. Predicted QALY gains per 10% improvement in SVR ranged from 0.23 (F0) to 0.64 (F4) and 0.58 (F0) to 1.35 (F4) in 40 year old patients (discounted and non-discounted results respectively). In those aged 40, projected discounted HCV-related costs are minimised with successful treatment in F0/F1 (at approximately £ 300), increasing to £ 49,300 in F4 patients who remain untreated. Validation of the model to published UK cost-effectiveness studies produce R2 goodness of fit statistics of 0.988, 0.978 and of 0.973 for total costs, QALYs and incremental cost effectiveness ratios, respectively.
Conclusion: Projecting the long-term clinical and economic consequences associated with chronic hepatitis C is a necessary requirement for the evaluation of new treatments. The principle analysis demonstrates the significant impact on expected costs, LYs and QALYs associated with increasing SVR. A validation analysis demonstrated the robustness of the results reported.
Conflict of interest statement
Figures




References
-
- Public Health England (2014). Hepatits C in the UK: 2014 report. Available: http://www.gov.uk/government/publications/hepatitis-c-in-the-uk. Accessed October 2014.
-
- Esteban JI, Sauleda S, Quer J (2008) The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol 48: 148–162. - PubMed
-
- Public Health England (2014). Commissioning template for estimating HCV prevalence by DAT and numbers eligible for treatment. Available: http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa..... Accessed October 2014.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

