Inflammation and ER stress regulate branched-chain amino acid uptake and metabolism in adipocytes
- PMID: 25635940
- PMCID: PMC4347289
- DOI: 10.1210/me.2014-1275
Inflammation and ER stress regulate branched-chain amino acid uptake and metabolism in adipocytes
Abstract
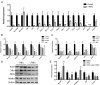
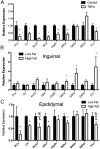
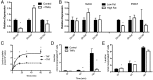
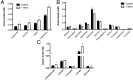
Inflammation plays a critical role in the pathology of obesity-linked insulin resistance and is mechanistically linked to the effects of macrophage-derived cytokines on adipocyte energy metabolism, particularly that of the mitochondrial branched-chain amino acid (BCAA) and tricarboxylic acid (TCA) pathways. To address the role of inflammation on energy metabolism in adipocytes, we used high fat-fed C57BL/6J mice and lean controls and measured the down-regulation of genes linked to BCAA and TCA cycle metabolism selectively in visceral but not in subcutaneous adipose tissue, brown fat, liver, or muscle. Using 3T3-L1 cells, TNFα, and other proinflammatory cytokine treatments reduced the expression of the genes linked to BCAA transport and oxidation. Consistent with this, [(14)C]-leucine uptake and conversion to triglycerides was markedly attenuated in TNFα-treated adipocytes, whereas the conversion to protein was relatively unaffected. Because inflammatory cytokines lead to the induction of endoplasmic reticulum stress, we evaluated the effects of tunicamycin or thapsigargin treatment of 3T3-L1 cells and measured a similar down-regulation in the BCAA/TCA cycle pathway. Moreover, transgenic mice overexpressing X-box binding protein 1 in adipocytes similarly down-regulated genes of BCAA and TCA metabolism in vivo. These results indicate that inflammation and endoplasmic reticulum stress attenuate lipogenesis in visceral adipose depots by down-regulating the BCAA/TCA metabolism pathway and are consistent with a model whereby the accumulation of serum BCAA in the obese insulin-resistant state is linked to adipose inflammation.
Figures


References
-
- Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006;580(12):2917–2921. - PubMed
-
- Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23(8):407–415. - PubMed
-
- Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314(1):1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

