Structure-guided design of a high affinity inhibitor to human CtBP
- PMID: 25636004
- PMCID: PMC4844192
- DOI: 10.1021/cb500820b
Structure-guided design of a high affinity inhibitor to human CtBP
Abstract
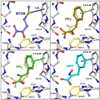
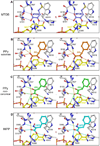
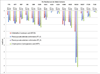
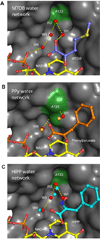
Oncogenic transcriptional coregulators C-terminal Binding Protein (CtBP) 1 and 2 possess regulatory d-isomer specific 2-hydroxyacid dehydrogenase (D2-HDH) domains that provide an attractive target for small molecule intervention. Findings that the CtBP substrate 4-methylthio 2-oxobutyric acid (MTOB) can interfere with CtBP oncogenic activity in cell culture and in mice confirm that such inhibitors could have therapeutic benefit. Recent crystal structures of CtBP 1 and 2 revealed that MTOB binds in an active site containing a dominant tryptophan and a hydrophilic cavity, neither of which are present in other D2-HDH family members. Here, we demonstrate the effectiveness of exploiting these active site features for the design of high affinity inhibitors. Crystal structures of two such compounds, phenylpyruvate (PPy) and 2-hydroxyimino-3-phenylpropanoic acid (HIPP), show binding with favorable ring stacking against the CtBP active site tryptophan and alternate modes of stabilizing the carboxylic acid moiety. Moreover, ITC experiments show that HIPP binds to CtBP with an affinity greater than 1000-fold over that of MTOB, and enzymatic assays confirm that HIPP substantially inhibits CtBP catalysis. These results, thus, provide an important step, and additional insights, for the development of highly selective antineoplastic CtBP inhibitors.
Figures



References
-
- Barroilhet L, Yang J, Hasselblatt K, Paranal RM, Ng SK, Rauh-Hain JA, Welch WR, Bradner JE, Berkowitz RS, Ng SW. C-terminal binding protein-2 regulates response of epithelial ovarian cancer cells to histone deacetylase inhibitors. Oncogene. 2013;32:3896–3903. - PubMed
-
- Di LJ, Byun JS, Wong MM, Wakano C, Taylor T, Bilke S, Baek S, Hunter K, Yang H, Lee M, Zvosec C, Khramtsova G, Cheng F, Perou CM, Ryan Miller C, Raab R, Olopade OI, Gardner K. Genome-wide profiles of CtBP link metabolism with genome stability and epithelial reprogramming in breast cancer. Nat Commun. 2013;4:1449. - PMC - PubMed
-
- Wang R, Asangani IA, Chakravarthi BV, Ateeq B, Lonigro RJ, Cao Q, Mani RS, Camacho DF, McGregor N, Schumann TE, Jing X, Menawat R, Tomlins SA, Zheng H, Otte AP, Mehra R, Siddiqui J, Dhanasekaran SM, Nyati MK, Pienta KJ, Palanisamy N, Kunju LP, Rubin MA, Chinnaiyan AM, Varambally S. Role of transcriptional corepressor CtBP1 in prostate cancer progression. Neoplasia. 2012;14:905–914. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

