Phenotypic profiling of CD8(+) T cells during Plasmodium vivax blood-stage infection
- PMID: 25636730
- PMCID: PMC4329216
- DOI: 10.1186/s12879-015-0762-x
Phenotypic profiling of CD8(+) T cells during Plasmodium vivax blood-stage infection
Abstract
Background: For a long time, the role of CD8(+) T cells in blood-stage malaria was not considered important because erythrocytes do not express major histocompatibility complex (MHC) class I proteins. While recent evidences suggest that CD8(+) T cells may play an important role during the erythrocytic phase of infection by eliminating parasites, CD8(+) T cells might also contribute to modulate the host response through production of regulatory cytokines. Thus, the role of CD8(+) T cells during blood-stage malaria is unclear. Here, we report the phenotypic profiling of CD8(+) T cells subsets from patients with uncomplicated symptomatic P. vivax malaria.
Methods: Blood samples were collected from 20 Plasmodium vivax-infected individuals and 12 healthy individuals. Immunophenotyping was conducted by flow cytometry. Plasma levels of IFN-γ, TNF-α and IL-10 were determined by ELISA/CBA. Unpaired t-test or Mann-Whitney test was used depending on the data distribution.
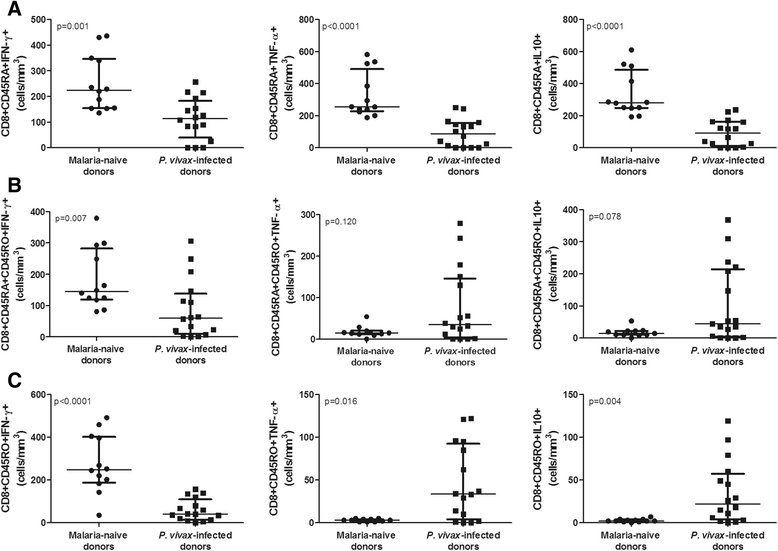
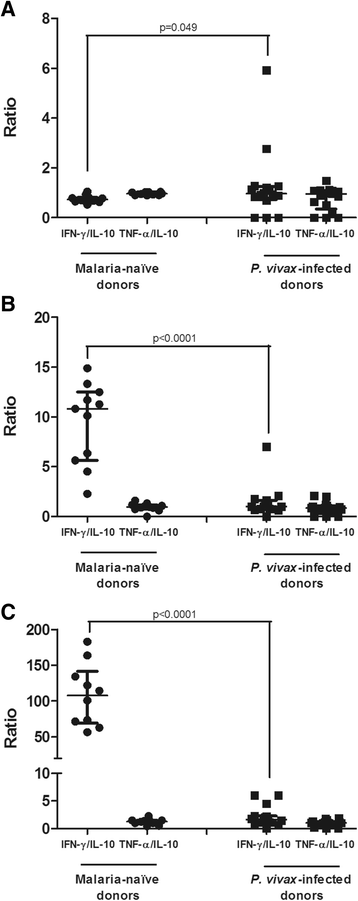
Results: P. vivax-infected subjects had lower percentages and absolute numbers of CD8(+)CD45RA(+) and CD8(+)CD45RO(+) T cells when compared to uninfected individuals (p ≤ 0.0002). A significantly lower absolute number of circulating CD8(+)CD45(+)CCR7(+) cells (p = 0.002) was observed in P. vivax-infected individuals indicating that infection reduces the number of central memory T cells. Cytokine expression was significantly reduced in the naïve T cells from infected individuals compared with negative controls, as shown by lower numbers of IFN-γ(+) (p = 0.001), TNF-α(+) (p < 0.0001) and IL-10(+) (p < 0.0001) CD8(+) T cells. Despite the reduction in the number of CD8(+) memory T cells producing IFN-γ (p < 0.0001), P. vivax-infected individuals demonstrated a significant increase in memory CD8(+)TNF-α(+) (p = 0.016) and CD8(+)IL-10(+) (p = 0.004) cells. Positive correlations were observed between absolute numbers of CD8(+)IL-10(+) and numbers of CD8(+)IFN-γ(+) (p < 0.001) and CD8(+)TNF-α(+) T cells (p ≤ 0.0001). Finally, an increase in the plasma levels of TNF-α (p = 0.017) and IL-10 (p = 0.006) and a decrease in the IFN-γ plasma level (p <0.0001) were observed in the P. vivax-infected individuals.
Conclusions: P. vivax infection reduces the numbers of different subsets of CD8(+) T cells, particularly the memory cells, during blood-stage of infection and enhances the number of CD8(+) memory T cells expressing IL-10, which positively correlates with the number of cells expressing TNF-α and IFN-γ.
Figures




References
-
- WHO. Country profile. In: World Malaria Report 2013. World Health Organization. 2013. http://www.who.int/malaria/publications/world_malaria_report_2013/en/. Accessed 18 Set 2014
-
- Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, Niangaly M, Dara C, Kayentao K, Ongoiba A, Doumbu OK, Traore B, Crompton PD. An intensive longitudinal cohort study of malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis. 2013;57(1):40–47. doi: 10.1093/cid/cit174. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

