Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin
- PMID: 25637025
- PMCID: PMC4337483
- DOI: 10.4049/jimmunol.1401064
Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin
Abstract
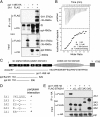
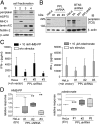
The three butyrophilin BTN3A molecules, BTN3A1, BTN3A2, and BTN3A3, are members of the B7/butyrophilin-like group of Ig superfamily receptors, which modulate the function of T cells. BTN3A1 controls activation of human Vγ9/Vδ2 T cells by direct or indirect presentation of self and nonself phosphoantigens (pAg). We show that the microbial metabolite (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate binds to the intracellular B30.2 domain of BTN3A1 with an affinity of 1.1 μM, whereas the endogenous pAg isopentenyl pyrophosphate binds with an affinity of 627 μM. Coculture experiments using knockdown cell lines showed that in addition to BTN3A1, BTN3A2 and BTN3A3 transmit activation signals to human γδ T cells in response to (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate and the aminobisphosphonate drug zoledronate that causes intracellular accumulation of isopentenyl pyrophosphate. The plakin family member periplakin, identified in yeast two-hybrid assays, interacted with a membrane-proximal di-leucine motif, located proximal to the B30.2 domain in the BTN3A1 cytoplasmic tail. Periplakin did not interact with BTN3A2 or BTN3A3, which do not contain the di-leucine motif. Re-expression into a BTN3A1 knockdown line of wild-type BTN3A1, but not of a variant lacking the periplakin binding motif, BTN3A1Δexon5, restored γδ T cell responses, demonstrating a functional role for periplakin interaction. These data, together with the widespread expression in epithelial cells, tumor tissues, and macrophages detected using BTN3A antiserum, are consistent with complex functions for BTN3A molecules in tissue immune surveillance and infection, linking the cell cytoskeleton to γδ T cell activation by indirectly presenting pAg to the Vγ9/Vδ2 TCR.
Copyright © 2015 The Authors.
Figures




References
-
- Willcox C. R., Mohammed F., Willcox B. E. 2013. Resolving the mystery of pyrophosphate antigen presentation. Nat. Immunol. 14: 886–887. - PubMed
-
- Eberl M., Hintz M., Reichenberg A., Kollas A. K., Wiesner J., Jomaa H. 2003. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 544: 4–10. - PubMed
-
- Riganti C., Massaia M., Davey M. S., Eberl M. 2012. Human γδ T-cell responses in infection and immunotherapy: common mechanisms, common mediators? Eur. J. Immunol. 42: 1668–1676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

