ANGPTL7 regulates the expansion and repopulation of human hematopoietic stem and progenitor cells
- PMID: 25637050
- PMCID: PMC4420207
- DOI: 10.3324/haematol.2014.118612
ANGPTL7 regulates the expansion and repopulation of human hematopoietic stem and progenitor cells
Abstract
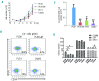
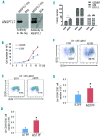
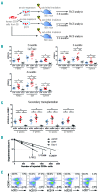
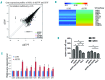
Successful expansion of hematopoietic stem cells would benefit the use of hematopoietic stem cell transplants in the clinic. Several angiopoietin-like proteins, including angiopoietin-like 7, can support the activity of hematopoietic stem cells. However, effects of ANGPTL7 on human hematopoietic stem cells and the downstream signaling cascade activated by ANGPTL7 are poorly understood. Here, we established a human hematopoietic stem and progenitor cell-supportive mouse fetal liver cell line that specifically expressed the Angptl7 protein. Furthermore, we found ANGPTL7 is capable of stimulating human hematopoietic stem and progenitor cell expansion and increasing the repopulation activities of human hematopoietic progenitors in xenografts. RNA-sequencing analysis showed that ANGPTL7 activated the expression of CXCR4, HOXB4 and Wnt downstream targets in human hematopoietic progenitors. In addition, chemical manipulation of Wnt signaling diminished the effects of ANGPTL7 on human hematopoietic stem and progenitor cells in culture. In summary, we identify the secreted growth factor ANGPTL7 as a regulator of both human hematopoietic stem and progenitor cell expansion and regeneration.
Copyright© Ferrata Storti Foundation.
Figures

Similar articles
-
Loss of Angiopoietin-like 7 diminishes the regeneration capacity of hematopoietic stem and progenitor cells.J Hematol Oncol. 2015 Feb 6;8:7. doi: 10.1186/s13045-014-0102-4. J Hematol Oncol. 2015. PMID: 25652910 Free PMC article.
-
Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells.Nat Med. 2006 Feb;12(2):240-5. doi: 10.1038/nm1342. Epub 2006 Jan 22. Nat Med. 2006. PMID: 16429146 Free PMC article.
-
Comparative integromics on Angiopoietin family members.Int J Mol Med. 2006 Jun;17(6):1145-9. Int J Mol Med. 2006. PMID: 16685428
-
Differential hematopoietic supportive potential and gene expression of stroma cell lines from midgestation mouse placenta and adult bone marrow.Cell Transplant. 2011;20(5):707-26. doi: 10.3727/096368910X536590. Epub 2010 Nov 5. Cell Transplant. 2011. PMID: 21054929
-
The role of Wnt signaling in hematopoietic stem cell development.Crit Rev Biochem Mol Biol. 2017 Aug;52(4):414-424. doi: 10.1080/10409238.2017.1325828. Epub 2017 May 16. Crit Rev Biochem Mol Biol. 2017. PMID: 28508727 Free PMC article. Review.
Cited by
-
Quantitative evaluation of the immunodeficiency of a mouse strain by tumor engraftments.J Hematol Oncol. 2015 May 29;8:59. doi: 10.1186/s13045-015-0156-y. J Hematol Oncol. 2015. PMID: 26022250 Free PMC article.
-
CRISPR/Cas9-Mediated Deletion of Foxn1 in NOD/SCID/IL2rg-/- Mice Results in Severe Immunodeficiency.Sci Rep. 2017 Aug 10;7(1):7720. doi: 10.1038/s41598-017-08337-8. Sci Rep. 2017. PMID: 28798321 Free PMC article.
-
Patient-Derived Xenograft Models in Cancer Research: Methodology, Applications, and Future Prospects.Methods Mol Biol. 2024;2806:9-18. doi: 10.1007/978-1-0716-3858-3_2. Methods Mol Biol. 2024. PMID: 38676792 Review.
-
Single-cell transcriptomic analysis of human pleura reveals stromal heterogeneity and informs in vitro models of mesothelioma.Eur Respir J. 2024 Jan 25;63(1):2300143. doi: 10.1183/13993003.00143-2023. Print 2024 Jan. Eur Respir J. 2024. PMID: 38212075 Free PMC article.
-
Coinhibition of activated p38 MAPKα and mTORC1 potentiates stemness maintenance of HSCs from SR1-expanded human cord blood CD34+ cells via inhibition of senescence.Stem Cells Transl Med. 2020 Dec;9(12):1604-1616. doi: 10.1002/sctm.20-0129. Epub 2020 Jun 29. Stem Cells Transl Med. 2020. PMID: 32602209 Free PMC article.
References
-
- Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453(7193):306–313. - PubMed
-
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8(4):290–301. - PubMed
-
- Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. - PubMed
-
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109(1):39–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

