Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks
- PMID: 25637061
- PMCID: PMC4335094
- DOI: 10.1007/s40261-014-0266-2
Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks
Abstract
Background and objectives: Long-term use of antiretroviral therapy (ART) to treat HIV infection has been associated with dyslipidemia and metabolic and cardiovascular complications. Available options for patients at risk of cardiovascular disease include antiretroviral drugs with improved lipid profiles. Dolutegravir is one of a new generation of HIV integrase inhibitors recently incorporated into the US Department of Health and Human Services, German, Spanish, and Italian HIV treatment guidelines as a preferred first-line third agent in combination with dual nucleoside reverse transcriptase inhibitor (NRTI) backbone therapies. To understand the lipid profile of dolutegravir in the context of combination ART, we analyzed the lipid outcomes at 48 weeks in ART-naive participants in four phase IIb-IIIb clinical trials.
Methods: Variables included in this analysis were total cholesterol (TC), low-density lipoprotein (LDL) cholesterol (LDL-C), high-density lipoprotein (HDL) cholesterol (HDL-C), TC/HDL ratio, and triglycerides at baseline and week 48.
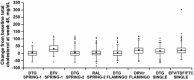
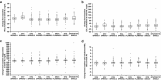
Results: In a comparative analysis, dolutegravir demonstrated a broadly neutral effect on lipids versus efavirenz or ritonavir-boosted darunavir; in both comparisons, patients taking dolutegravir exhibited smaller increases in TC, LDL-C, and triglyceride levels. In comparison with raltegravir, dolutegravir exhibited a similar lipid profile, including small increases in TC, LDL-C, and triglyceride levels for both agents. In the pooled dolutegravir analysis, minimal increases in LDL-C and triglycerides were observed but mean values at 48 weeks remained below National Cholesterol Education Program target levels. HDL-C levels increased at 48 weeks, and the mean TC/HDL-C ratio was 0.6 at 48 weeks; these values are associated with a lower risk of cardiovascular disease.
Conclusions: Together, these data show that dolutegravir has a safer lipid profile in combination ART and provides an important treatment option for older patients who may have other risk factors for metabolic syndrome or cardiovascular disease.
Figures

References
-
- Raffi F, Jaeger H, Quiros-Roldan E, Albrecht H, Belonosova E, Gatell JM, Extended SPRING-2 study group et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13:927–935. doi: 10.1016/S1473-3099(13)70257-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

