The dose-effect safety profile of skeletal muscle precursor cell therapy in a dog model of intrinsic urinary sphincter deficiency
- PMID: 25637189
- PMCID: PMC4339845
- DOI: 10.5966/sctm.2014-0114
The dose-effect safety profile of skeletal muscle precursor cell therapy in a dog model of intrinsic urinary sphincter deficiency
Abstract
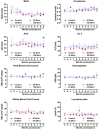
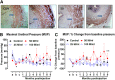
Locally injected skeletal muscle precursor cells (skMPCs) integrate into and restore the muscle layers, innervation, vasculature, and function of the sphincter complex in animal models of intrinsic urinary sphincter deficiency (ISD). The goal of the present study was to test the dose-effect safety profile of skMPC therapy in a dog model of ISD. Sphincter deficiency was created in 20 adult female dogs by surgically removing the skeletal muscle layer of the urinary sphincter complex. skMPCs isolated from the hind leg were expanded in culture and injected 4 weeks later into the sphincter complex at a dose of 25 million cells (n = 5), 50 million cells (n = 5), or 100 million cells (n = 5) per milliliter in a 2-ml volume. Five dogs received no sphincter injection. The measures of maximal sphincter pressure, complete blood count, and blood chemistry were performed monthly until their sacrifice at 9 months. At that point, full necropsy was performed to assess the safety of the skMPC injections. Injection of different doses of cells had no effects on the body weight, blood cell count, or kidney or liver function test results (p > .05 among the skMPC doses). Some incidental pathologic features were found in the lower urinary tract in all groups and were most likely associated with repeat catheterization. The maximal urinary sphincter pressure was higher in the 50 million cells per milliliter treatment group than in the other experimental groups (p < .05). The findings of the present study have confirmed that urinary sphincter injection of skMPCs results in no significant local or systemic pathologic features within the dose range that improves sphincter pressures.
Keywords: Maximal urethral pressure; Pathology; Skeletal muscle; Stem cells; Urinary incontinence.
©AlphaMed Press.
Figures


Similar articles
-
Local versus intravenous injections of skeletal muscle precursor cells in nonhuman primates with acute or chronic intrinsic urinary sphincter deficiency.Stem Cell Res Ther. 2016 Oct 7;7(1):147. doi: 10.1186/s13287-016-0411-3. Stem Cell Res Ther. 2016. PMID: 27717380 Free PMC article.
-
Determinates of muscle precursor cell therapy efficacy in a nonhuman primate model of intrinsic urinary sphincter deficiency.Stem Cell Res Ther. 2017 Jan 6;8(1):1. doi: 10.1186/s13287-016-0461-6. Stem Cell Res Ther. 2017. PMID: 28057078 Free PMC article.
-
Efficacy and Initial Safety Profile of CXCL12 Treatment in a Rodent Model of Urinary Sphincter Deficiency.Stem Cells Transl Med. 2017 Aug;6(8):1740-1746. doi: 10.1002/sctm.16-0497. Epub 2017 Jul 17. Stem Cells Transl Med. 2017. PMID: 28714578 Free PMC article.
-
[Intrinsic sphincter deficiency and female urinary incontinence].Prog Urol. 2015 Jun;25(8):437-54. doi: 10.1016/j.purol.2015.03.006. Epub 2015 Apr 9. Prog Urol. 2015. PMID: 25864653 Review. French.
-
Urinary incontinence: sphincter functioning from a urological perspective.Digestion. 2004;69(2):93-101. doi: 10.1159/000077875. Epub 2004 Apr 14. Digestion. 2004. PMID: 15087576 Review.
Cited by
-
3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration.Sci Rep. 2018 Aug 17;8(1):12307. doi: 10.1038/s41598-018-29968-5. Sci Rep. 2018. PMID: 30120282 Free PMC article.
-
A double-blind, randomized, placebo-controlled clinical trial evaluating the safety and efficacy of autologous muscle derived cells in female subjects with stress urinary incontinence.Int Urol Nephrol. 2018 Dec;50(12):2153-2165. doi: 10.1007/s11255-018-2005-8. Epub 2018 Oct 15. Int Urol Nephrol. 2018. PMID: 30324580 Clinical Trial.
-
Stem Cells for Urinary Incontinence: Functional Differentiation or Cytokine Effects?Urology. 2018 Jul;117:9-17. doi: 10.1016/j.urology.2018.01.002. Epub 2018 Jan 12. Urology. 2018. PMID: 29339111 Free PMC article. Review.
-
Intraurethral co-transplantation of bone marrow mesenchymal stem cells and muscle-derived cells improves the urethral closure.Stem Cell Res Ther. 2018 Sep 21;9(1):239. doi: 10.1186/s13287-018-0990-2. Stem Cell Res Ther. 2018. PMID: 30241573 Free PMC article.
-
Development of self-nanoemulsifying drug delivery system for oral bioavailability enhancement of valsartan in beagle dogs.Drug Deliv Transl Res. 2017 Feb;7(1):100-110. doi: 10.1007/s13346-016-0342-7. Drug Deliv Transl Res. 2017. PMID: 27812915
References
-
- Holroyd-Leduc JM, Straus SE. Management of urinary incontinence in women: Scientific review. JAMA. 2004;291:986–995. - PubMed
-
- Brown JS, Nyberg LM, Kusek JW, et al. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases International Symposium on Epidemiologic Issues in Urinary Incontinence in Women. Am J Obstet Gynecol. 2003;188:S77–S88. - PubMed
-
- Hannestad YS, Rortveit G, Sandvik H, et al. A community-based epidemiological survey of female urinary incontinence: The Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150–1157. - PubMed
-
- Wilson L, Brown JS, Shin GP, et al. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

