The dangers of dental devices as reported in the Food and Drug Administration Manufacturer and User Facility Device Experience Database
- PMID: 25637208
- PMCID: PMC4313571
- DOI: 10.1016/j.adaj.2014.11.015
The dangers of dental devices as reported in the Food and Drug Administration Manufacturer and User Facility Device Experience Database
Abstract
Background: The authors conducted a study to determine the frequency and type of adverse events (AEs) associated with dental devices reported to the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database.
Methods: The authors downloaded and reviewed the dental device-related AEs reported to MAUDE from January 1, 1996, through December 31,2011.
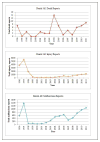
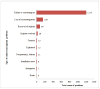
Results: MAUDE received a total of 1,978,056 reports between January 1, 1996, and December 31, 2011. Among these reports, 28,046 (1.4%) AE reports were associated with dental devices. Within the dental AE reports that had event type information, 17,261 reported injuries, 7,777 reported device malfunctions, and 66 reported deaths. Among the 66 entries classified as death reports, 52 reported a death in the description; the remaining were either misclassified or lacked sufficient information in the report to determine whether a death had occurred. Of the dental device-associated AEs, 53.5% pertained to endosseous implants.
Conclusions: A plethora of devices are used in dental care. To achieve Element 1 of Agency for Healthcare Research and Quality's Patient Safety Initiative, clinicians and researchers must be able to monitor the safety of dental devices. Although MAUDE was identified by the authors as essentially the sole source of this valuable information on adverse events, their investigations led them to conclude that MAUDE had substantial limitations that prevent it from being the broad-based patient safety sentinel the profession requires.
Practical implications: As potential contributors to MAUDE, dental care teams play a key role in improving the profession's access to information about the safety of dental devices.
Keywords: Dental equipment; dental public health; dental records; informatics; quality of care; safety management.
Copyright © 2015 American Dental Association. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- What is a Serious Adverse Event? 2014 Jun 16; Available from: http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.htm.
-
- Ramoni RB, Walji MF, White J, Stewart D, Vaderhobli R, Simmons D, et al. From good to better: Toward a patient safety initiative in dentistry. Journal of the American Dental Association (1939) 2012;143(9):956–60. - PubMed
-
-
AHRQ’s Patient Safety Initiative: Building Foundations, Reducing Risk. Interim Report to the Senate Committee on Appropriations. 2003 December. Report No.: Contract No.: AHRQ Publication No. 04-RG005,.
-
-
- Classification of Products as Drugs and Devices and Additional Product Classification Issues. 2014 Jun 15; Available from: http://www.fda.gov/RegulatoryInformation/Guidances/ucm258946.htm-_Toc294....
-
- Class 2 Device Recall CollaPlug Absorbable Collagen Wound Dressing for Dental Surgery. 2014 Jun 15; Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=117050.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials