Systemic Agonistic Anti-CD40 Treatment of Tumor-Bearing Mice Modulates Hepatic Myeloid-Suppressive Cells and Causes Immune-Mediated Liver Damage
- PMID: 25637366
- PMCID: PMC4420683
- DOI: 10.1158/2326-6066.CIR-14-0182
Systemic Agonistic Anti-CD40 Treatment of Tumor-Bearing Mice Modulates Hepatic Myeloid-Suppressive Cells and Causes Immune-Mediated Liver Damage
Abstract
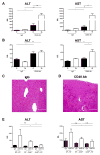
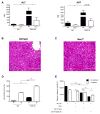
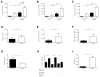
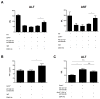
Immune-stimulatory mAbs are currently being evaluated as antitumor agents. Although overall toxicity from these agents appears to be moderate, liver toxicities have been reported and are not completely understood. We studied the effect of systemic CD40 antibody treatment on myeloid cells in the spleen and liver. Naïve and tumor-bearing mice were treated systemically with agonistic anti-CD40 antibody. Immune cell subsets in the liver and spleen, serum transaminases, and liver histologies were analyzed after antibody administration. Nox2(-/-), Cd40(-/-), and bone marrow chimeric mice were used to study the mechanism by which agonistic anti-CD40 mediates its effects in vivo. Suppressor function of murine and human tumor-induced myeloid-derived suppressor cells (MDSC) was studied upon CD40 ligation. Agonistic CD40 antibody caused liver damage within 24 hours after injection in two unrelated tumor models and mice strains. Using bone marrow chimeras, we demonstrate that CD40 antibody-induced hepatitis in tumor-bearing mice was dependent on the presence of CD40-expressing hematopoietic cells. Agonistic CD40 ligation-dependent liver damage was induced by the generation of reactive oxygen species. Furthermore, agonistic CD40 antibody resulted in increased CD80-positive and CD40-positive liver CD11b(+)Gr-1(+) immature myeloid cells. CD40 ligation on tumor-induced murine and human CD14(+)HLA-DR(low) peripheral blood mononuclear cells from patients with cancer reduced their immune suppressor function. Collectively, agonistic CD40 antibody treatment activated tumor-induced myeloid cells, caused myeloid-dependent hepatotoxicity, and ameliorated the suppressor function of murine and human MDSC. Collectively, our data suggest that CD40 may mature immunosuppressive myeloid cells and thereby cause liver damage in mice with an accumulation of tumor-induced hepatic MDSC.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures

Similar articles
-
Tumor-induced CD11b(+) Gr-1(+) myeloid-derived suppressor cells exacerbate immune-mediated hepatitis in mice in a CD40-dependent manner.Eur J Immunol. 2015 Apr;45(4):1148-58. doi: 10.1002/eji.201445093. Epub 2015 Feb 23. Eur J Immunol. 2015. PMID: 25616156 Free PMC article.
-
Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells.Cancer Immunol Immunother. 2014 Aug;63(8):847-57. doi: 10.1007/s00262-014-1561-8. Epub 2014 May 31. Cancer Immunol Immunother. 2014. PMID: 24878890 Free PMC article.
-
Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer.Cancer Res. 2010 Jan 1;70(1):99-108. doi: 10.1158/0008-5472.CAN-09-1882. Epub 2009 Dec 8. Cancer Res. 2010. PMID: 19996287 Free PMC article.
-
Myeloid derived suppressor cells in human diseases.Int Immunopharmacol. 2011 Jul;11(7):802-7. doi: 10.1016/j.intimp.2011.01.003. Epub 2011 Jan 13. Int Immunopharmacol. 2011. PMID: 21237299 Free PMC article. Review.
-
The Use of Anti-CD40 mAb in Cancer.Curr Top Microbiol Immunol. 2017;405:165-207. doi: 10.1007/82_2014_427. Curr Top Microbiol Immunol. 2017. PMID: 25651948 Review.
Cited by
-
Injectable Nanoparticle-Based Hydrogels Enable the Safe and Effective Deployment of Immunostimulatory CD40 Agonist Antibodies.Adv Sci (Weinh). 2022 Oct;9(28):e2103677. doi: 10.1002/advs.202103677. Epub 2022 Aug 17. Adv Sci (Weinh). 2022. PMID: 35975424 Free PMC article.
-
Serum Amyloid A Proteins and Their Impact on Metastasis and Immune Biology in Cancer.Cancers (Basel). 2021 Jun 25;13(13):3179. doi: 10.3390/cancers13133179. Cancers (Basel). 2021. PMID: 34202272 Free PMC article. Review.
-
Cellular based immunotherapy for primary liver cancer.J Exp Clin Cancer Res. 2021 Aug 9;40(1):250. doi: 10.1186/s13046-021-02030-5. J Exp Clin Cancer Res. 2021. PMID: 34372912 Free PMC article. Review.
-
The Role of Myeloid Cells in Hepatotoxicity Related to Cancer Immunotherapy.Cancers (Basel). 2022 Apr 10;14(8):1913. doi: 10.3390/cancers14081913. Cancers (Basel). 2022. PMID: 35454819 Free PMC article. Review.
-
A versatile pretargeting approach for tumour-selective delivery and activation of TNF superfamily members.Sci Rep. 2017 Oct 16;7(1):13301. doi: 10.1038/s41598-017-13530-w. Sci Rep. 2017. PMID: 29038485 Free PMC article.
References
-
- Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical Activity and Immune Modulation in Cancer Patients Treated With CP-870,893, a Novel CD40 Agonist Monoclonal Antibody. Journal of Clinical Oncology. 2007;25:876–83. - PubMed
-
- Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. - PubMed
-
- Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11:91–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

