Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome
- PMID: 25637548
- PMCID: PMC4385996
- DOI: 10.1152/ajpheart.00779.2014
Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome
Abstract
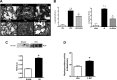
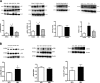
Fifty percent of trauma patients who present sepsis-like syndrome do not have bacterial infections. This condition is known as systemic inflammatory response syndrome (SIRS). A unifying factor of SIRS and sepsis is cardiovascular collapse. Trauma and severe blood loss cause the release of endogenous molecules known as damage-associated molecular patterns. Mitochondrial N-formyl peptides (F-MIT) are damage-associated molecular patterns that share similarities with bacterial N-formylated peptides and are potent immune system activators. The goal of this study was to investigate whether F-MIT trigger SIRS, including hypotension and vascular collapse via formyl peptide receptor (FPR) activation. We evaluated cardiovascular parameters in Wistar rats treated with FPR or histamine receptor antagonists and inhibitors of the nitric oxide pathway before and after F-MIT infusion. F-MIT, but not nonformylated peptides or mitochondrial DNA, induced severe hypotension via FPR activation and nitric oxide and histamine release. Moreover, F-MIT infusion induced hyperthermia, blood clotting, and increased vascular permeability. To evaluate the role of leukocytes in F-MIT-induced hypotension, neutrophil, basophil, or mast cells were depleted. Depletion of basophils, but not neutrophils or mast cells, abolished F-MIT-induced hypotension. Rats that underwent hemorrhagic shock increased plasma levels of mitochondrial formylated proteins associated with lung damage and antagonism of FPR ameliorated hemorrhagic shock-induced lung injury. Finally, F-MIT induced vasodilatation in isolated resistance arteries via FPR activation; however, F-MIT impaired endothelium-dependent relaxation in the presence of blood. These data suggest that F-MIT may be the link among trauma, SIRS, and cardiovascular collapse.
Keywords: cardiovascular collapse; mitochondrial N-formyl peptides; sepsis-like syndrome.
Copyright © 2015 the American Physiological Society.
Figures





References
-
- Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med 29, Suppl: S109–S116, 2001. - PubMed
-
- Cohen J. The immunopsthogenesis of sepsis. Nature 420: 885–891, 2002. - PubMed
-
- de Paulis A, Prevete N, Fiorentino I, Walls AF, Curto M, Petraroli A, Castaldo V, Ceppa P, Fiocca R, Marone G. Basophils infiltrate human gastric mucosa at sites of Helicobacter pylori infection, and exhibit chemotaxis in response to H. pylori-derived peptide Hp(2–20). J Immunol 172: 7734–7743, 2004. - PubMed
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

