Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn's-like murine ileitis
- PMID: 25637591
- PMCID: PMC4438744
- DOI: 10.1189/jlb.3HI0614-303R
Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn's-like murine ileitis
Abstract
The regulation of T cell and DC retention and lymphatic egress within and from the intestine is critical for intestinal immunosurveillance; however, the cellular processes that orchestrate this balance during IBD remain poorly defined. With the use of a mouse model of TNF-driven Crohn's-like ileitis (TNF(Δ) (ARE)), we examined the role of CCR7 in the control of intestinal T cell and DC retention/egress during experimental CD. We observed that the frequency of CCR7-expressing TH1/TH17 effector lymphocytes increased during active disease in TNF(Δ) (ARE) mice and that ΔARE/CCR7(-/-) mice developed exacerbated ileitis and multiorgan inflammation, with a marked polarization and ileal retention of TH1 effector CD4(+) T cells. Furthermore, adoptive transfer of ΔARE/CCR7(-/-) effector CD4(+) into lymphopenic hosts resulted in ileo-colitis, whereas those transferred with ΔARE/CCR7(+/+) CD4(+) T cells developed ileitis. ΔARE/CCR7(-/-) mice had an acellular draining MLN, decreased CD103(+) DC, and decreased expression of RALDH enzymes and of CD4(+)CD25(+)FoxP3(+) Tregs. Lastly, a mAb against CCR7 exacerbated ileitis in TNF(Δ) (ARE) mice, phenocopying the effects of congenital CCR7 deficiency. Our data underscore a critical role for the lymphoid chemokine receptor CCR7 in orchestrating immune cell traffic and TH1 versus TH17 bias during chronic murine ileitis.
Keywords: CD103+ dendritic cells; Crohn’s disease; effector memory T cells; inflammatory bowel disease; lymphatics.
© Society for Leukocyte Biology.
Figures
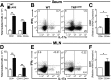
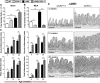
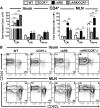
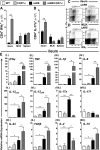

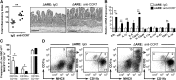

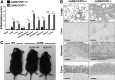
Comment in
-
Editorial: CCR7 is required for leukocyte egression in an experimental model of Crohn's disease-like ileitis.J Leukoc Biol. 2015 Jun;97(6):1000-2. doi: 10.1189/jlb.5CE0215037RR. J Leukoc Biol. 2015. PMID: 26031490 No abstract available.
References
-
- Veny M., Esteller M., Ricart E., Piqué J. M., Panés J., Salas A. (2010) Late Crohn’s disease patients present an increase in peripheral Th17 cells and cytokine production compared with early patients. Aliment. Pharmacol. Ther. 31, 561–572. - PubMed
-
- Sakuraba A., Sato T., Kamada N., Kitazume M., Sugita A., Hibi T. (2009) Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology 137, 1736–1745. - PubMed
-
- Bromley S. K., Thomas S. Y., Luster A. D. (2005) Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6, 895–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

