Truncated somatostatin receptor variant sst5TMD4 confers aggressive features (proliferation, invasion and reduced octreotide response) to somatotropinomas
- PMID: 25637790
- PMCID: PMC4378269
- DOI: 10.1016/j.canlet.2015.01.037
Truncated somatostatin receptor variant sst5TMD4 confers aggressive features (proliferation, invasion and reduced octreotide response) to somatotropinomas
Abstract
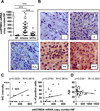
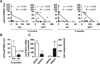
The GH/IGF1 response of somatotropinomas to somatostatin analogues (SSA) is associated with their pattern of somatostatin receptor (sst1-sst5) expression. Recently, we demonstrated that expression of a truncated sst5-variant (sst5TMD4) can influence the secretory response of somatotropinomas to SSA-therapy; however, its potential relationship with aggressive features (e.g. invasion/proliferation) is still unknown. Here, we show that sst5TMD4 is present in 50% of non-functioning pituitary-adenomas (NFPA) (n = 30) and 89% of somatotropinomas (n = 36), its expression levels being highest in somatotropinomas > > NFPAs > > > normal pituitaries (negligible expression; n = 8). In somatotropinomas, sst5TMD4 mRNA and protein levels correlated positively, and its expression was directly associated with tumor invasiveness (cavernous/sphenoid sinus), and inversely correlated with age and GH/IGF1 reduction after 3-6 months with octreotide-LAR therapy. GNAS+ somatotropinomas expressed lower sst5TMD4 levels. ROC analysis revealed sst5TMD4 expression as the only marker, within all sst-subtypes, capable to predict tumor invasiveness in somatotropinomas. sst5TMD4 overexpression increased cell viability in cultured somatotropinoma (n = 5). Hence, presence of sst5TMD4 associates with increased aggressive features and worse prognosis in somatotropinomas, thereby providing a potentially useful tool to refine somatotropinoma diagnosis, predict outcome of clinical response to SSA-therapy and develop new therapeutic targets.
Keywords: Acromegaly; Invasion; Proliferation; sst5TMD4.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
We wish to draw attention to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work.
The rest of the authors have nothing to disclose.
Figures


References
-
- Theodoropoulou M, Stalla GK. Somatostatin receptors: from signaling to clinical practice. Front. Neuroendocrinol. 2013;34:228–252. - PubMed
-
- Colao A, Auriemma RS, Lombardi G, Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr. Rev. 2011;32:247–271. - PubMed
-
- Cuevas-Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. J. Mol. Endocrinol. 2014;52:R223–R240. - PubMed
-
- Giustina A, Mazziotti G, Maffezzoni F, Amoroso V, Berruti A. Investigational drugs targeting somatostatin receptors for treatment of acromegaly and neuroendocrine tumors. Expert Opin. Investig. Drugs. 2014;23:1619–1635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

