Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes
- PMID: 25637831
- PMCID: PMC4696552
- DOI: 10.1016/j.mam.2015.01.003
Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes
Abstract
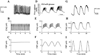
Type 2 diabetes (T2DM) results when increases in beta cell function and/or mass cannot compensate for rising insulin resistance. Numerous studies have documented the longitudinal changes in metabolism that occur during the development of glucose intolerance and lead to T2DM. However, the role of changes in insulin secretion, both amount and temporal pattern, has been understudied. Most of the insulin secreted from pancreatic beta cells of the pancreas is released in a pulsatile pattern, which is disrupted in T2DM. Here we review the evidence that changes in beta cell pulsatility occur during the progression from glucose intolerance to T2DM in humans, and contribute significantly to the etiology of the disease. We review the evidence that insulin pulsatility improves the efficacy of secreted insulin on its targets, particularly hepatic glucose production, but also examine evidence that pulsatility alters or is altered by changes in peripheral glucose uptake. Finally, we summarize our current understanding of the biophysical mechanisms responsible for oscillatory insulin secretion. Understanding how insulin pulsatility contributes to normal glucose homeostasis and is altered in metabolic disease states may help improve the treatment of T2DM.
Keywords: Diabetes; Insulin pulsatility; Insulin resistance; Islets; Oscillations.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

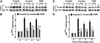




References
-
- Ward WK, Beard JC, Porte D., Jr Clinical aspects of islet B-cell function in non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1986;2:297–313. - PubMed
-
- Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes. 2002;51(Suppl 1):S53–S59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

