Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure: the supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial
- PMID: 25637937
- PMCID: PMC4466154
- DOI: 10.1093/eurheartj/ehu504
Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure: the supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial
Abstract
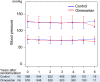
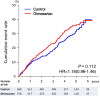
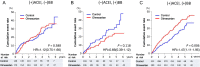
We examined whether an additive treatment with an angiotensin receptor blocker, olmesartan, reduces the mortality and morbidity in hypertensive patients with chronic heart failure (CHF) treated with angiotensin-converting enzyme (ACE) inhibitors, β-blockers, or both. In this prospective, randomized, open-label, blinded endpoint study, a total of 1147 hypertensive patients with symptomatic CHF (mean age 66 years, 75% male) were randomized to the addition of olmesartan (n = 578) to baseline therapy vs. control (n = 569). The primary endpoint was a composite of all-cause death, non-fatal acute myocardial infarction, non-fatal stroke, and hospitalization for worsening heart failure. During a median follow-up of 4.4 years, the primary endpoint occurred in 192 patients (33.2%) in the olmesartan group and in 166 patients (29.2%) in the control group [hazard ratio (HR) 1.18; 95% confidence interval (CI), 0.96-1.46, P = 0.112], while renal dysfunction developed more frequently in the olmesartan group (16.8 vs. 10.7%, HR 1.64; 95% CI 1.19-2.26, P = 0.003). Subgroup analysis revealed that addition of olmesartan to combination of ACE inhibitors and β-blockers was associated with increased incidence of the primary endpoint (38.1 vs. 28.2%, HR 1.47; 95% CI 1.11-1.95, P = 0.006), all-cause death (19.4 vs. 13.5%, HR 1.50; 95% CI 1.01-2.23, P = 0.046), and renal dysfunction (21.1 vs. 12.5%, HR 1.85; 95% CI 1.24-2.76, P = 0.003). Additive use of olmesartan did not improve clinical outcomes but worsened renal function in hypertensive CHF patients treated with evidence-based medications. Particularly, the triple combination therapy with olmesartan, ACE inhibitors and β-blockers was associated with increased adverse cardiac events. This study is registered at clinicaltrials.gov-NCT00417222.
Keywords: Angiotensin II receptor blocker; Heart failure; Hypertension; Olmesartan.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
Comment in
-
Too much is too much: evidence against dual RAAS inhibition in hypertensives with heart failure symptoms.Eur Heart J. 2015 Apr 14;36(15):899-901. doi: 10.1093/eurheartj/ehv033. Epub 2015 Feb 8. Eur Heart J. 2015. PMID: 25666318 No abstract available.
-
Heart failure: New data do not SUPPORT triple RAAS blockade.Nat Rev Nephrol. 2015 May;11(5):260-2. doi: 10.1038/nrneph.2015.30. Epub 2015 Mar 24. Nat Rev Nephrol. 2015. PMID: 25802078
References
-
- WRITING COMMITTEE MEMBERS Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240–e327. - PubMed
-
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. Erratum in Eur Heart J 2013;34:158. - PubMed
-
- Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K, CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772–776. - PubMed
-
- Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–1675. - PubMed
-
- McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA, CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767–771. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

