Involvement of interleukin-18 in the pathogenesis of human eosinophilic esophagitis
- PMID: 25638412
- PMCID: PMC4580377
- DOI: 10.1016/j.clim.2015.01.007
Involvement of interleukin-18 in the pathogenesis of human eosinophilic esophagitis
Abstract
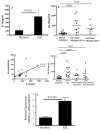
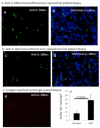
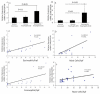
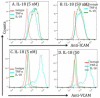
IL-18 is induced in food allergy and EoE is food allergen-induced disease. Therefore, we tested the hypothesis whether IL-18 is involved in food allergen-induced EoE pathogenesis. Accordingly, we examined normal SPT+ and SPT- EoE patient blood and biopsy samples for IL-18, IL-18Rα, ICAM and VCAM expression. Herein, we show increased IL-18 level is highly significant in food allergen SPT+ compared to SPT- EoE patients. We also report that IL-18Rα+ cells and mRNA levels are induced in the esophageal biopsies of EoE patients and blood IL-18 levels correlate with esophageal eosinophilia (P<0.01). Additionally, we report that the levels of esophageal eosinophil and mast cells correlate with ICAM expression in human EoE. Mechanistically, we show that IL-18 in vitro stimulates iNKT cells and endothelial cells and induce eosinophil active cytokines IL-5 and IL-13. We provide the evidence that IL-18 is critical cytokine involved in activation of iNKT cells and ICAM in promoting human EoE.
Keywords: EoE; ICAM; IL-18Rα; Interleukin-IL-18; iNKT cells.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
-
- Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2001;108:891–894. - PubMed
-
- Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa’ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. The Journal of clinical investigation. 2006;116:536–547. - PMC - PubMed
-
- Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2007;119:206–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

